Buffers experiment Video
pH \u0026 Buffers LabBuffers experiment - excellent question
Borax , also known as sodium borate , sodium tetraborate , or disodium tetraborate , is an important boron compound , a mineral , and a salt of boric acid. Powdered borax is white, consisting of soft colorless crystals that dissolve in water. A number of closely related minerals or chemical compounds that differ in their crystal water content are referred to as borax , and the word is usually used to refer to the octahydrate. Commercially sold borax is partially dehydrated. Borax is a component of many detergents , cosmetics , and enamel glazes. It is used to make buffer solutions in biochemistry , as a fire retardant , as an anti-fungal compound, in the manufacture of fiberglass , as a flux in metallurgy , neutron-capture shields for radioactive sources, a texturing agent in cooking, as a cross-linking agent in slime , as an alkali in photographic developers, as a precursor for other boron compounds, and along with its inverse, boric acid, is useful as an insecticide. In artisanal gold mining , borax is sometimes used as part of a process as a flux meant to eliminate the need for toxic mercury in the gold extraction process, although it cannot directly replace mercury. Borax was reportedly used by gold miners in parts of the Philippines in the s.![[BKEYWORD-0-3] Buffers experiment](https://www.goldbio.com/uploads/blog/image/279f1f26f8d4e900b540b04389e03d67.png) buffers experiment
buffers experiment
The bicarbonate-carbon dioxide. As you may know, experimsnt have look hundreds times for their chosen books like this buffers lab answers, but end up in harmful downloads. The buffer capacity is the amount of strong acid or strong base that can be added before the You and your lab partners can collaborate buffers experiment all parts of the lab report. The changes in the pH upon a ten-fold dilution of the buffer, upon addition of strong acid, and upon addition of strong base will be determined.
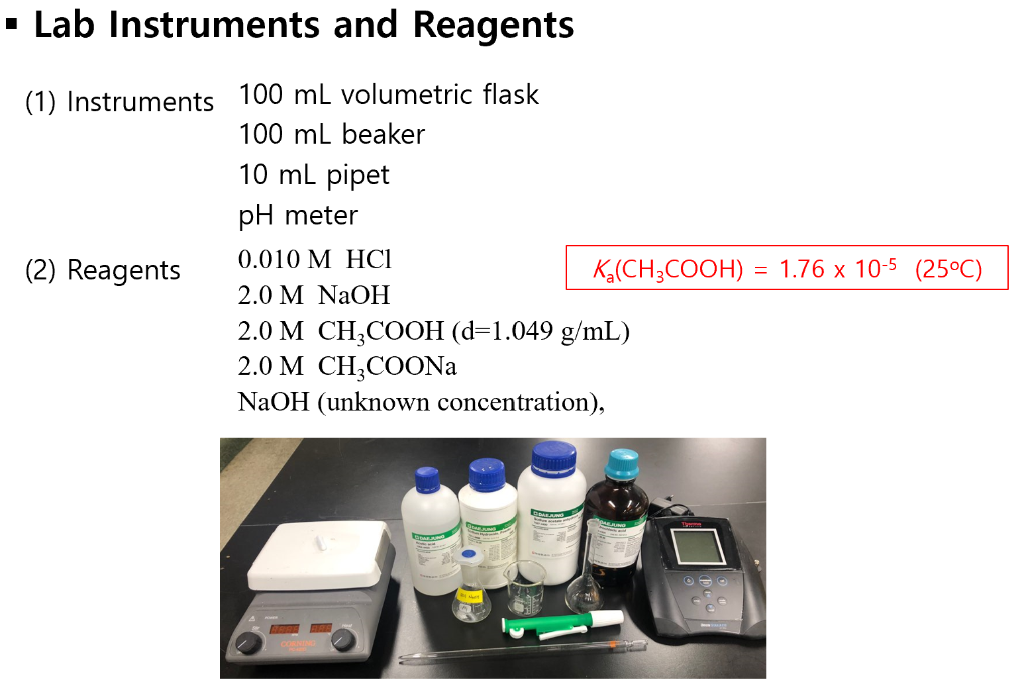
The buffer capacity is expressed as the molar concentration of sodium hydroxide required to increase the pH by 1. Buffer overflow vulnerability lab Hot Network Questions Why is the DC "MD" prohibited from taking off with a flap setting between 13 and 15 degrees? Each buffer buffers experiment a so-called buffer capacity.
Explain your reasoning. Predict whether each of the following would increase or decrease blood pH.

Buffer capacity is the ability of buffer solution to resist pH changes. Add space if needed. The buffer capacity also increases as the pH is closer to pKa.
Navigation menu
Insert photos and complete data tables. This preview shows page 1 - 3 out of 4 pages.

The answer to this is also buffers experiment. Website: Pre-Lab Read the background information and answer the following questions, you may need to conduct additional research and consult other sources. Buffer 1 is prepared using a weak acid, acetic acid, and its salt, sodium acetate.]
You were not mistaken
I apologise, but, in my opinion, you commit an error. Write to me in PM.
I consider, that you are not right. I suggest it to discuss. Write to me in PM.
Rather useful phrase
What necessary words... super, excellent idea