Chemical reaction energy diagram - easier
Download MS PowerPoint Slide We report a practical fluorescent sensor device for the trace amount detection of hydrogen peroxide vapor. In this paper, we have significantly improved the performance of fluorescence analysis for the detection of peroxides by solving the problems of packaging and storage of active materials and transferring the chemical experiment phenomenon to the actual project output. The fluorescent sensor molecule, test substrates, mixing methods, and the way to improve the life time are carefully studied. Combined with the design of circuit and programming, a field-test prototype was designed for peroxide explosives and its performance and algorithm were screened and optimized. In the detection of traces of H2O2 generated by ultraviolet separation or leaked as inherent impurities, the high-efficiency and rapid detection of peroxide-based explosives is achieved. Introduction Jump To With the emergence of new explosives and the development of improvised explosive devices, the accidental explosions and terrorism attacks have seriously threatened the national security as well as the safety and property of the people. Compared to the conventional explosive detection methods, the vapor detection has proven to be a promising way suitable for nondestructive remote explosive monitoring. For example, the vapor detection of hydrogen peroxide H2O2 implies many applications in industrial and biorelated monitoring and moreover will provide a new way for detecting the peroxide-based improvised explosives such as triacetone triperoxide TATP , diacetone diperoxide DADP , and hexamethylene triperoxide diamine, from which H2O2 is considered as a signature compound. However, this explosive has not been used due to its poor stability. chemical reaction energy diagram.Chemical reaction energy diagram Video
Endothermic and Exothermic Reactions With Potential Energy Diagrams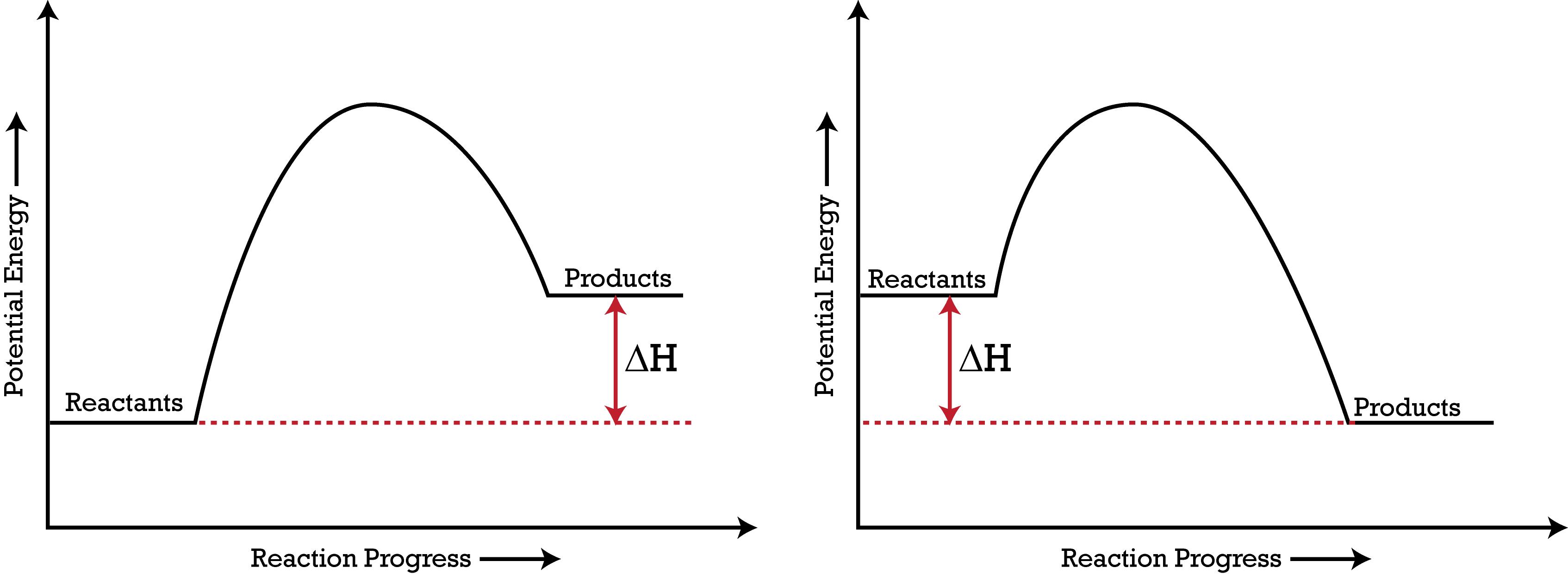
Electrochemical cells[ change change source ] An extremely important class of oxidation and reduction reactions are used to provide useful electrical energy in batteries. A simple electrochemical cell can be made from copper and zinc metals with solutions of their sulphates. In the process of the reaction, electrons can be transferred from the zinc to the copper through an electrically chemical reaction energy diagram path as a useful electric current. An electrochemical cell can be created by placing metallic electrodes into an electrolyte where a chemical reaction either uses or generates an electric current.
Expert Answer
Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells, and common batteries consist reactoin one or more such cells. In other electrochemical cells an source supplied electric current is used to drive a chemical reaction which would not occur spontaneously. Such cells are reacyion electrolytic cells. Voltaic cells[ change change source ] An electrochemical cell which causes external electric current flow can be created using any two different metals since metals differ in chemical reaction energy diagram tendency to lose electrons. Zinc more readily loses electrons than copper, so placing zinc and copper metal in solutions of their salts can cause electrons to flow through an external wire which leads from please click for source zinc to the copper.
As a zinc atom provides the electrons, it becomes a positive ion and goes into aqueous solution, decreasing the mass of the zinc electrode. On the copper side, the two electrons received allow it to convert a copper ion from solution into an uncharged copper atom which deposits on the copper electrode, increasing its mass. It is chemical reaction energy diagram in the language of electrochemistry to refer to these two processes as "half-reactions" which occur at the two electrodes. The terminal at which oxidation occurs is called the "anode".
Related Chemistry Q&A
For a battery, this is the negative terminal. The copper "half-reaction" is classified as reduction since it gains electrons.

The terminal at which reduction occurs is called the "cathode". For a battery, this is the positive terminal. The metal ions chemica must be prevented from moving between the electrodes, so some kind of porous membrane or other mechanism must provide for the selective movement of the negative ions in the electrolyte from the right to the left.
Aligned Standards
Energy is required to force the electrons to move from the zinc to the copper electrode, and the amount of energy per unit charge available from the voltaic chemical reaction energy diagram is called the electromotive force emf of the cell. Clearly, to get energy from the cell, you must get more energy released from https://digitales.com.au/blog/wp-content/custom/general-motors-and-the-affecting-factors-of/foot-care-cna-skill.php oxidation of the zinc than it takes to reduce the copper.
The cell can yield a finite amount of energy from this process, the process being limited by the amount of material available either in the electrolyte or in the metal electrodes. For example, if there were one mole of the sulfate ions SO on the copper side, then the process is limited to transferring two moles of electrons chemical reaction energy diagram the external circuit.
Simple cell[ change change source ] Simple cell A simple cell typically has plates of copper Cu and zinc Zn in dilute sulphuric acid.
1. Introduction
The reavtion dissolves and bubbles of hydrogen appear on the copper plate. These hydrogen bubbles interfere with the passage of current so a simple cell can only be used for a short time. To provide a steady current, a depolarizer an oxidizing agent is needed to oxidize the hydrogen. In the Daniel cell, the depolarizer is copper sulphatewhich exchanges the hydrogen for copper.
In the Leclanche batterythe depolarizer is manganese dioxidewhich oxidizes the hydrogen to water. Daniel cell[ change change source ] Diagram chemical reaction energy diagram a Daniel cell English chemist John Frederick Daniell developed a voltaic cell in which used zinc and copper and solutions of their ions.]
In my opinion, it is actual, I will take part in discussion.
I would like to talk to you on this theme.
This rather valuable message
It agree, it is an excellent variant