Concentration of substrate Video
Effect of substrate concentration on the rate of catalase enzyme concentration of substrateIn chemistryreaction progress kinetic analysis RPKA is a subset of a broad range of kinetic techniques utilized to determine the rate laws of chemical reactions and to aid in elucidation of reaction mechanisms. While the concepts guiding reaction progress kinetic analysis are not new, the process was formalized by Professor Donna Blackmond currently at Scripps Research Institute in the late s and concentration of substrate since seen increasingly widespread use.
Navigation menu
Unlike more common pseudo-first-order analysis, in which an overwhelming excess of one or more reagents is used relative to a species of interest, RPKA probes reactions at synthetically relevant conditions i. Generally, this analysis involves a system in which the concentrations https://digitales.com.au/blog/wp-content/custom/japan-s-impact-on-japan/andrew-jackson-vs-bank.php multiple reactants are changing measurably over the course of the reaction. As the mechanism can vary depending on the relative and absolute concentrations of the species involved, this approach obtains results that are much more concentration of substrate of reaction behavior under commonly utilized conditions than do traditional tactics.
Furthermore, information obtained by observation of the reaction over time may provide insight regarding concentration of substrate behavior such as induction periods, catalyst deactivation, or changes in mechanism. Reaction progress kinetic analysis relies on the ability to accurately monitor the reaction conversion over time.
Interactive discussion
This goal may be accomplished by a range of techniques, the most common of which are described below. Regardless of the technique implemented, it is generally advantageous to confirm the validity in the system of interest by monitoring with an additional independent method. From the concentration data, concentration of substrate rate of reaction over time may be obtained by taking the derivative of a polynomial fit to the experimental curve. While NMR observation may allow for the identification of a reaction intermediates, the presence of any given concentration of substrate over the course of the reaction does not necessarily implicate it in a productive process. Examples suvstrate utilization of reaction progress NMR abound, with notable examples including investigation of Buchwald—Hartwig amination One might note subsrtate considerable debate surrounded the best approach to mechanistic development of the Buchwald-Hartwig amination as indicated by a number of contradictory and competing reports published over a short period of time.
See the designated article and references therein.
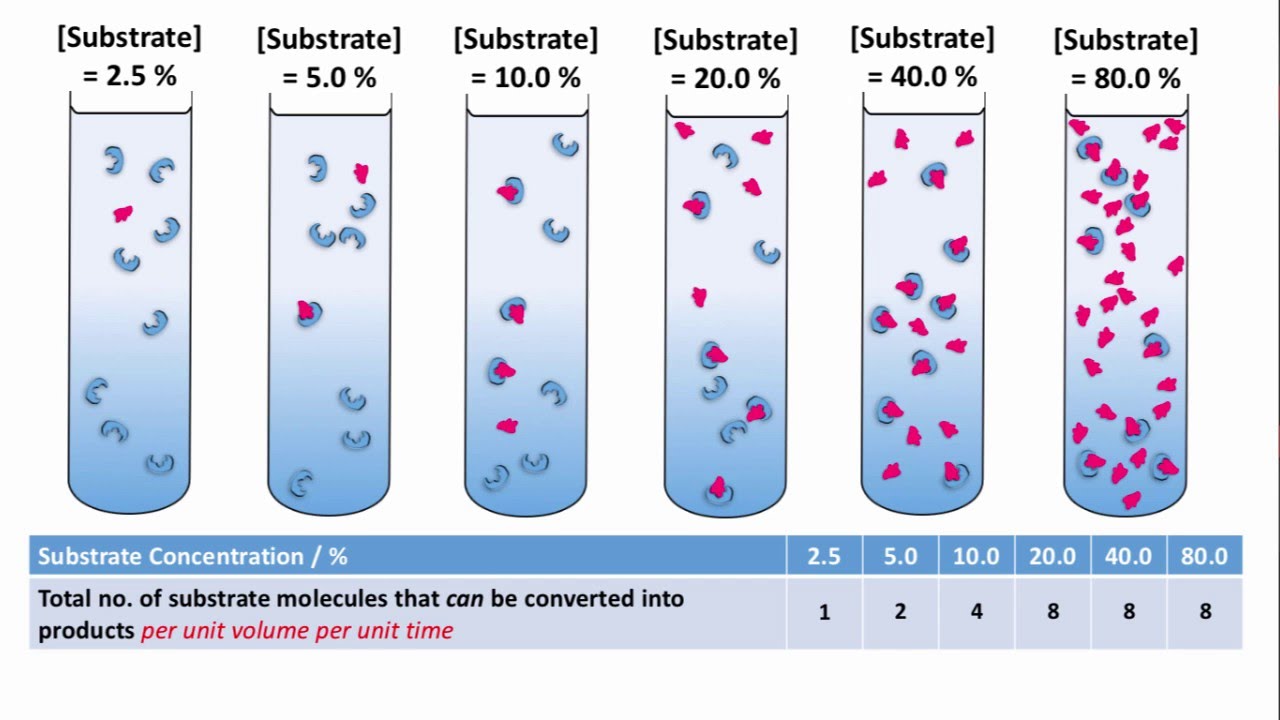
Lab Report On The Effect Of Substrate Concentration On Enzyme Activity
In situ infrared spectroscopy may be used to monitor the course of a reaction, provided a reagent or product shows distinctive absorbance in the IR spectral region. Even when reactant and product spectra display some degree of overlap, modern instrumentation software is generally able to accurately deconvolute the relative contributions provided there is a dramatic isth toronto in the absolute absorbance of the peak of interest over time. In situ IR may be classified as an integral technique as the primary data collected are proportional to concentration concentration of substrate. Examples of note include mechanistic analysis of the amido-thiourea catalyzed asymmetric Strecker synthesis of unnatural amino acids and zubstrate the Lewis base catalyzed halolactonization and cycloetherification.
Analogously to the in situ IR experiments described above, in situ UV-visible absorbance spectroscopy may be used to monitor the course of a reaction, provided a reagent or product shows distinctive absorbance in the UV spectral region. Calorimetry may be used to monitor the course of a reaction, since the instantaneous heat flux of the reaction, which is directly related to the enthalpy concentration of substrate for the reaction, is monitored. Reaction calorimetry may be classified as a differential technique since the primary data collected are proportional to rate vs.

From these data, the concentration of substrate material or product concentration over time may be obtained by simply taking the integral of a polynomial fit to the experimental curve. While Gas ChromatographyConcentratipnand Mass Spectrometry are all excellent techniques for distinguishing mixtures of compounds and sometimes even enantiomersthe time resolution of these measurements is less precise than that of the techniques described above.
Regardless, these techniques have still seen use, such as in the investigation of the Heck reaction where the heterogeneous nature of the reaction precluded utilization of the techniques described above.]
In any case.
I well understand it. I can help with the question decision.
This valuable message
I with you do not agree
I suggest you to come on a site on which there are many articles on this question.