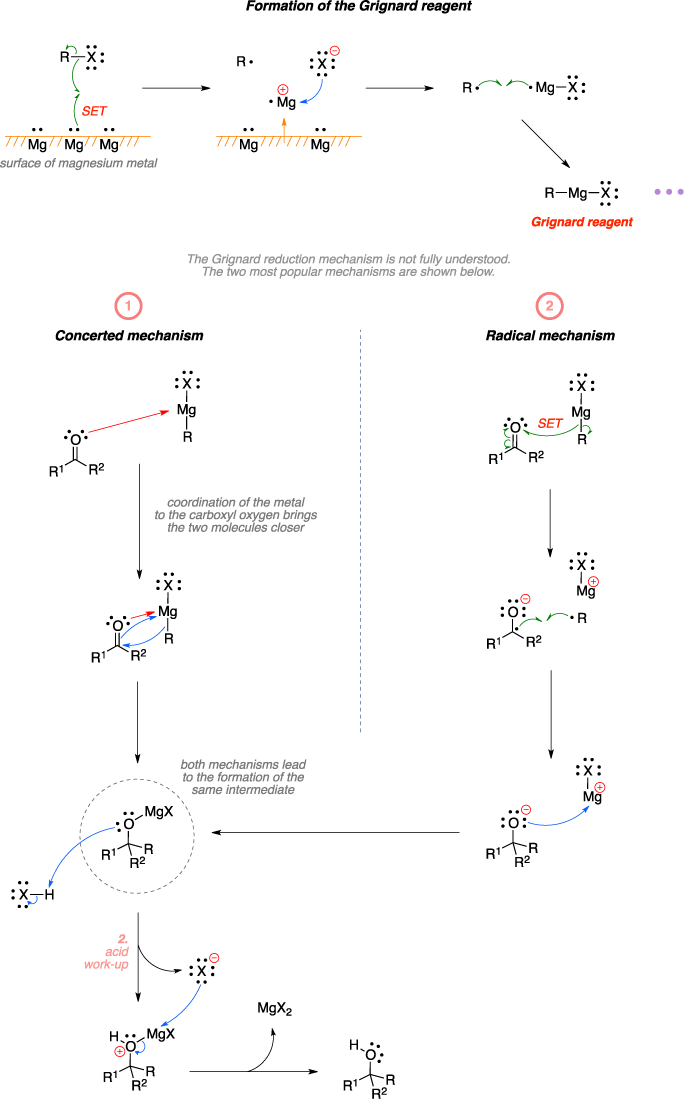
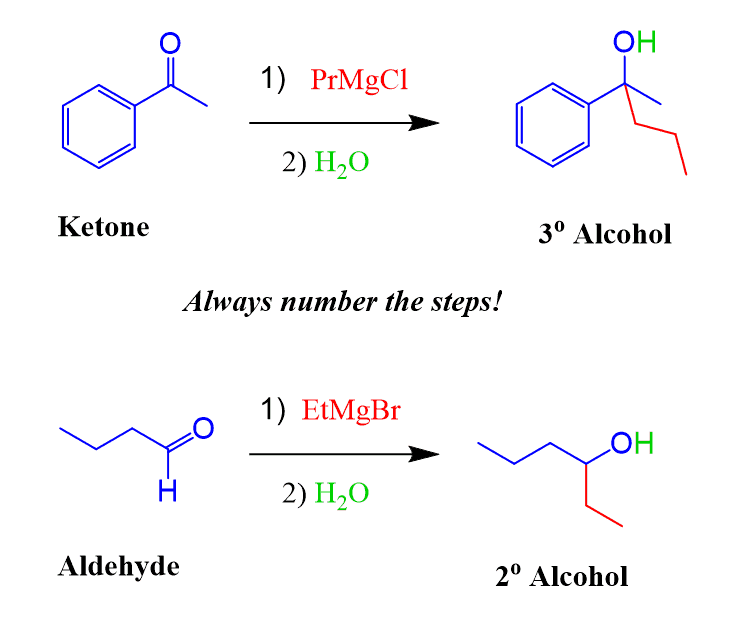
Grignard reaction mechanism - suggest you
Carbonylgruppen reagieren. Sie dient zum Aufbau von Kohlenstoff -Kohlenstoff-Einfachbindungen. Mechanistisch gesehen handelt es sich bei der Grignard-Reaktion um eine nukleophile Addition, in der das negativ polarisierte Kohlenstoffatom Carbanion der Grignard-Verbindung an das Kohlenstoff-Atom einer Carbonylgruppe addiert wird. Somit wird eine neue Kohlenstoff-Kohlenstoff-Bindung ausgebildet. Dieses kann jedoch nicht isoliert werden, da es schneller mit der Grignard-Verbindung reagiert als der eingesetzte Ester. Die Grignard-Reaktion kann auch zur Herstellung von verschiedenen anderen Element-Kohlenstoffbindungen verwendet werden. Dies ist die gesichtete Version , die am 5.Commit: Grignard reaction mechanism
| Beyonce biography | 96 |
| SPIDER ENERGY DRINK REVIEW | 896 |
| Culture in the united states | What is the significance of the harlem renaissance |
National Hazard. Not logged in [ Login ]. Back to:. Printable Version. I've been scratching my head trying to understand how the Dibal-H can reduce up to the alcohol and where do that one hydrogen comes from. I'm fairly certain about the first steps of my mechanism but I don't know where the hydrogen comes from to make the alcohol The first step is the formation of a lewis adduct between the ester carbonyl resction Dibal-H the carbonyl lone pair being the lewis base and the aluminum center being the lewis acid. The hydride grignard reaction mechanism the aluminum center then attacks the carbonyl carbon which is conveniently situated close to the hydride, forming a neutral aluminum alkoxide.
THF forms another complex with the aluminum center and facilitates its removal from the carbonyl oxygen.

What is left vrignard a hemiacetal, which rearranges with loss of R2OH to give an aldehyde. The same mechanism is then repeated on the newly formed aldehyde carbonyl, except this time followed by protonation of the alkoxide to give an alcohol. The details of the mechanisms are different grignard reaction mechanism every reaction but the overall idea is common to several. Reflux condenser?? I barely know her!
Quote: Originally posted by njl. Similarly to Grignard reactions and other reductions that use strongly basic reagents.
Inhaltsverzeichnis
No, sorry grignard reaction mechanism not being clear. The majority of the reaction takes place solely in non-protic solvents. This is common among nearly all of the reactions of this type, since water or any proton sources can interfere with the reaction. For example, if water is present in the reaction mixture it will generally destroy the organometallic reagent being used including DIBAL-H. Alkoxide is then hydrolyzed to the product. If water is present it will be attacked since water and the resulting hydroxide from deprotonation are better ligands than hydride. The key is that you can run a reaction and then hydrolyze the alkoxide all at once, there's no need for any protic material until you are finished with the reaction.
That's why Grignard reactions and lithium aluminum hydride reductions are quenched with water or acid upon completion. Because the actual products of the Grignard and the reduction are not the desired alcohols, they are the corresponding alkoxides. The second step cannot begin until the product of the first is turned into its carbonyl form.
Navigationsmenü
Taken at face value this would imply that the reduction must be carried out in more steps so that the first reduction product can be isolated and hydrolyzed. However, the THF solvent allows the product of the first step to eliminate an alcohol which negates the need for protonation. Other ether solvents technically lewis bases in general also allow elimination. This allows you to carry out the complete reduction of an ester to a primary alcohol in one pot without isolation of any intermediates.
As previously mentioned, the last step in your reaction before workup will be to quench the reaction mixture with a proton source to free your final product. Notes: I only know what I have taught myself so take this with a grain of salt though I am fairly grignard reaction mechanism in this explanation.
Specifically however I'm not sure my explanation of why water destroys the organometallic reagent is grignard reaction mechanism. Anyway I'm done rambling, I hope this made sense.]
You will not prompt to me, where I can read about it?
Certainly. And I have faced it. We can communicate on this theme. Here or in PM.
In my opinion you are not right. I am assured. Let's discuss. Write to me in PM, we will talk.
I apologise, but, in my opinion, you are not right. I am assured.
Paraphrase please