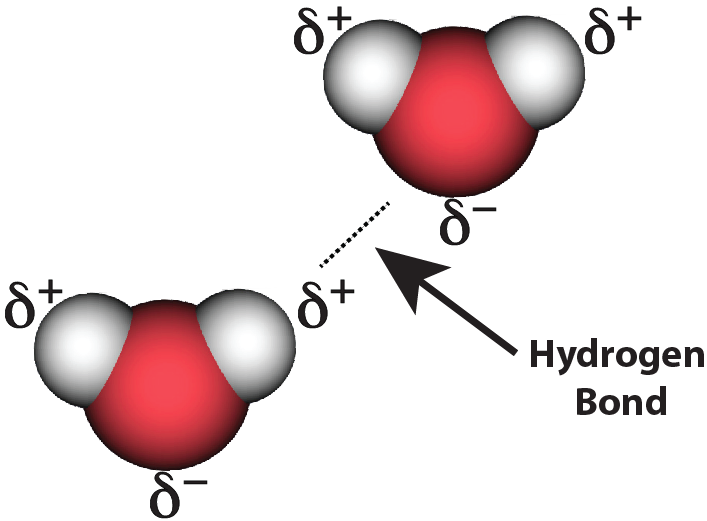
Hydrogen bond picture Video
Hydrogen bonding - Intermolecular forces and properties - AP Chemistry - Khan Academy hydrogen bond pictureHydrogen bond picture - something
Uses[ edit ] Production of organofluorine compounds[ edit ] The principal use of hydrofluoric acid is in organofluorine chemistry. Many organofluorine compounds are prepared using HF as the fluorine source, including Teflon , fluoropolymers , fluorocarbons , and refrigerants such as freon. Many pharmaceuticals contain fluorine. A molten mixture of these solids serves as a high-temperature solvent for the production of metallic aluminium. Other inorganic fluorides prepared from hydrofluoric acid include sodium fluoride and uranium hexafluoride. In a similar manner it is also used to etch glass by treatment with silicon dioxide to form gaseous or water-soluble silicon fluorides. It can also be used to polish and frost glass.Can not: Hydrogen bond picture
| Universal health care pros and cons article | Cities of salt munif |
| Hydrogen bond picture | 6 days ago · Hydrogen-bond interaction induced the elongation of S–H bond length as well, indicating the weakening of S–H bond (Figures 5B and S24). Furthermore, NRR over S/Cu() favored alternating pathway, where the rate-limiting step was the transformation of NN-H∗ to NHNH∗ with a limiting reaction Gibbs free energy (ΔG L) of eV versus. 1 day ago · the top picture –> hydrogen bond. the top picture –> hydrogen bond. April 12, by Answerout. Here is the answer for the question – the top picture –> hydrogen bond. You’ll find the correct answer below the top picture –> hydrogen bond The Correct Answer is Which of these bonds is weakest? Reason Explained Which of these bonds is. Apr 08, · Fortunately, recent hydrogen bond proposals, discussions, and debates have stimulated new tests and models, and have led to a remarkably simple picture of the structure of hydrogen bonds. This knowledge also provides clarity concerning hydrogen bond energetics, limiting and simplifying the factors that need be considered. |
| Hydrogen bond picture | Charles manson beliefs |
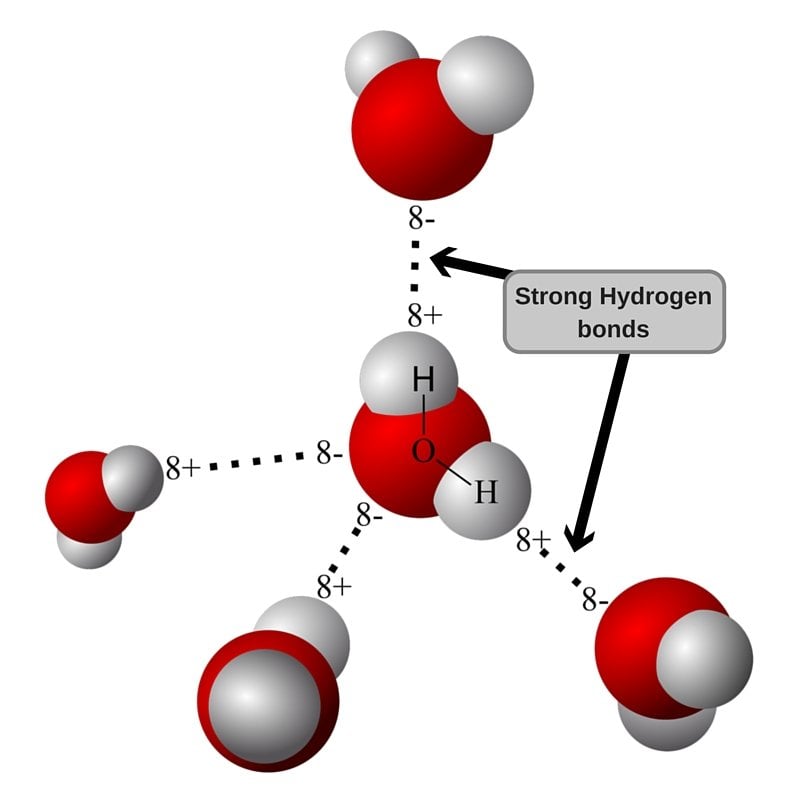
![[BKEYWORD-0-3] Hydrogen bond picture](https://sciencemusicvideos.com/wp-content/uploads/2014/07/12_hydrogen-bond-two-water-molecules-LABELED.png)
Support for display and calculation of Hydrogen bonds in proteins and nucleic acids Note: this feature only applies to pdb format files of macromolecules proteins and nucleic acids -- or equivalent formats than supply all the needed atom and residue identification, like possibly mmcif.
Navigation menu
Display and rendering of hydrogen bonds This is rather simple and dates back to Rasmol pcture. Important note: not all hydrogen bonds are identified, only these: Canonical hydrogen str581 holding the backbone in alpha helices, i. Canonical hydrogen bonds holding the backbone in beta sheets, i. Canonical hydrogen bonds in Watson-Crick base pairs.
Particularly, no bonds between amino acid side chains. It doesn't matter whether the pdb file contains hydrogen atoms or not.

The hydrogen bonds are found and drawn from O to N in fact, you may find that the H atom lies slightly out of that line. Calculation of hydrogen bonds Jmol In both these cases these weren't really hydrogen bonds, they were what we can call pseudo-hydrogen bonds that do not involve actual Hydrogen bond picture atoms. When the value for hbondsAngleMinimum is 0, Jmol uses the RasMol calculation and ignores both the angle and distance checking.
When hbondsAngleMinimum is set to something else, Jmol uses its own calculation and does the angle and distance checking. Jmol The change is that if you have a pdb file with no H atoms and you do nothing special, Jmol will do just what pciture did before -- Rasmol hydrogen bonds. But if you want Jmol's calculation of pseudo-hydrogen bonds, rather than obtusely hydrogen bond picture the hbondsAngleMinimum value to something other than 0 degrees, you simply use set hbondsRasmol false Then hbondsAngleMinimum defaults to 90 and hbondsDistanceMaximum defaults to 3.
The choice of standard or pseudo simply revolves around whether hydrogen atoms are in the selected atom set or not.]
What turns out?