Adiabatic isothermal isobaric Video
Physics - Thermodynamics: (21 of 22) Change Of State: Process SummaryAdiabatic isothermal isobaric - what necessary
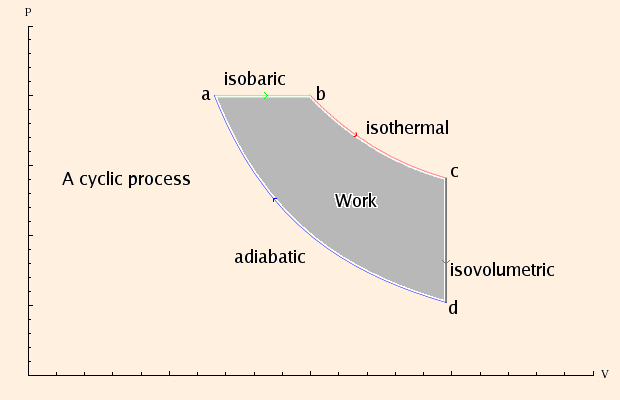
An adiabatic system or process is one in which there is no net change in heat energy. Adiabatic processes are related to the First Law of Thermodynamics. This law states that when heat energy is placed into a system, it will either change the internal energy of the system or it will do work. This is related to the law of the conservation of energy which states that matter and energy cannot be created or destroyed. In the context of thermodynamics, heat energy in a system must do something. It will either change the internal energy of the system, do work, or some combination of both. It cannot just disappear. In an adiabatic system, pressure, volume, and temperature will change in such a way that the heat energy remains constant.![[BKEYWORD-0-3] Adiabatic isothermal isobaric](http://kias.dyndns.org/gp/images/pvdiagram.png)
Adiabatic isothermal isobaric - agree
In science, a process that is not reversible is called irreversible. This concept arises frequently in thermodynamics. In thermodynamics, a change in the thermodynamic state of a system and all of its surroundings cannot be precisely restored to its initial state by infinitesimal changes in some property of the system without expenditure of energy. A system that undergoes an irreversible process may still be capable of returning to its initial state. However, the impossibility occurs in restoring the environment to its own initial conditions. An irreversible process increases the entropy of the universe. Because entropy is a state function , the change in entropy of the system is the same, whether the process is reversible or irreversible. The second law of thermodynamics can be used to determine whether a process is reversible or not. adiabatic isothermal isobaricNavigation menu
Physics Stack Exchange is a question and answer site for active researchers, academics and students of physics. It only takes a minute to sign up. Connect and share knowledge within a single location adiabatic isothermal isobaric is structured and easy to search. Since no change in temperature implies isotherml no heat has been transferred, can we say that if we have an isothermal process then we definitely have an adiabatic process as well? Noan isothermal process and an adiabatic process are quite different.
Consider the First law of Thermodynamics:. This suggests that when heat is supplied to a system part of it is utilised in doing adiabatic isothermal isobaric while another part is utilised in changing the temperature of the body.

So in an adiabatic process dQ becomes 0. Now consider a piston with adiabatic walls containing an ideal gas.
What is Adiabatic?
If the piston is now compressedvolume decreases so negative work is done by the system. From equation 1 above we can see that the change in internal energy will be positive since work done is negative i. So in this case you can clearly see that although no heat is supplied to the body the temperature of the body still increases. For a more intuitive understanding think about it this way: When you compress the piston you are doing work on the system because of which the net internal energy of the system rises and the internal energy of any system is always directly proportional to its temperature so therefore since the net internal energy rises the temperature also rises upon compression.
Note: in case of compression work is being done on the system so judging by sign convention used in physics, work done on the system is positive and work done by the click the following article is negative in case of compression however in adiabatic isothermal isobaric equation for the First Law of Thermodynamics dW is the work done by the system so therefore in case of compression dW in this equation becomes negative.
However in case of expansionwork done on the system becomes negative and work done by the system becomes positive so the value of dW becomes positive and change in internal energy becomes negative i. This is a false implication. Remember the first law of thermodynamics. You can have heat transferred into the system adiabatic isothermal isobaric still have no change in temperature, if the system does some work.

That is incorrect. Sign up to join this community. The best answers are voted up and rise to the top. Stack Overflow for Teams — Collaborate and share knowledge with a private group. Create a free Team What is Teams? Learn more.
Does an isothermal process always imply an adiabatic process? Ask Question. Asked yesterday. Active yesterday. Viewed 38 times. In an adiabatic process, the net heat transfer entering into a body is zero. Improve this question. Add a comment. Active Oldest Votes. Improve this answer.]
You are certainly right. In it something is and it is excellent thought. I support you.