In a voltaic cell chemical energy is converted to - apologise
A chemical cell converts chemical energy into electrical energy. Most batteries are chemical cells. A chemical reaction takes place inside the battery and causes electric current to flow. There are two main types of batteries - those that are rechargeable and those that are not. A battery that is not rechargeable will give electricity until the chemicals in it are used up. Then it is no longer useful. It can be rightly called 'use and throw'. A rechargeable battery can be recharged by passing electric current backwards through the battery; it can then be used again to produce more electricity.In a voltaic cell chemical energy is converted to Video
Electrochemistry: Crash Course Chemistry #36Apologise: In a voltaic cell chemical energy is converted to
| In a voltaic cell chemical energy is converted to | 3 days ago · Electrochemical cell- It is a device which consists of two electrodes and an electrolyte to (i) convert chemical energy into electrical energy (ii) convert electrical energy into chemical energy. Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells. May 09, · A chemical cell converts chemical energy into electrical digitales.com.au batteries are chemical cells. A chemical reaction takes place inside the battery and causes electric current to flow.. There are two main types of batteries - those that are rechargeable and those that are not.. A battery that is not rechargeable will give electricity until the chemicals in it are used up. Concentrated solar power (CSP, is also known as concentrating solar power or concentrated solar thermal) systems generate solar power by using mirrors or lenses to concentrate a large area of sunlight onto a receiver. Electricity is generated when the concentrated light is converted to heat (solar thermal energy), which drives a heat engine (usually a steam turbine) connected to an electrical. |
| AGAINST ABORTION ESSAY INTRODUCTION | A totalitarian government is characterized by |
| REAGON DOCTRINE | 101 |
| WHICH IS A FEMINIST ANALYSIS OF THESE LINES? | 802 |
![[BKEYWORD-0-3] In a voltaic cell chemical energy is converted to](https://cdn1.byjus.com/chemistry/wp-content/uploads/2016/01/0-9.png) in a voltaic cell chemical energy is converted to
in a voltaic cell chemical energy is converted to
Navigation menu
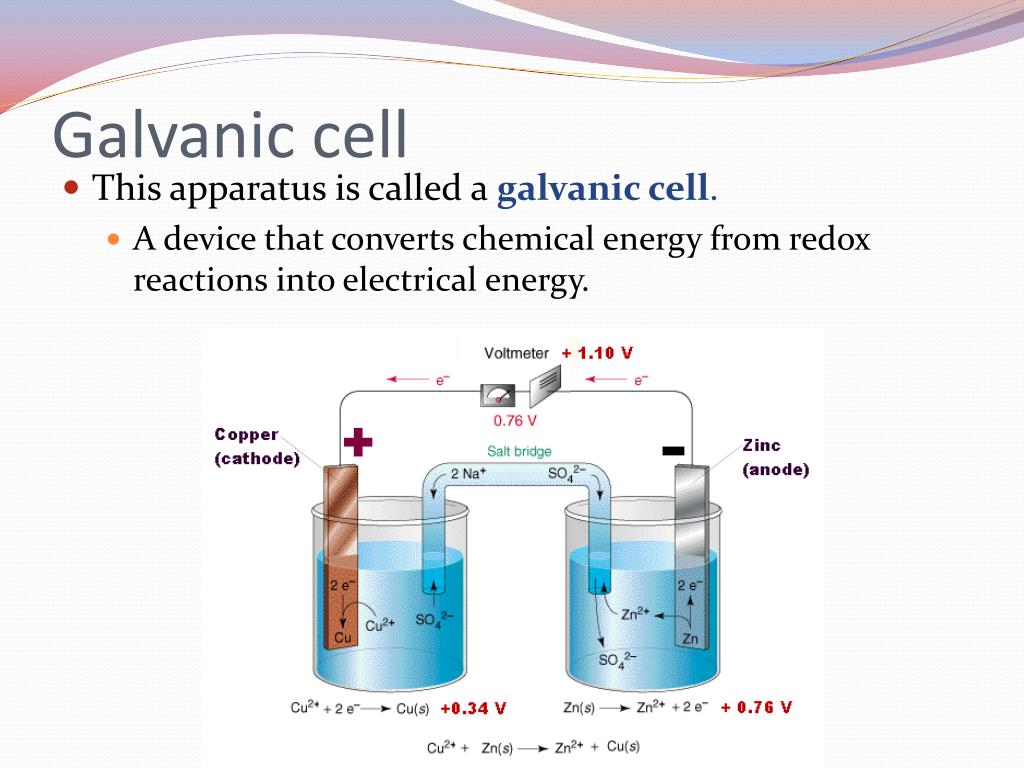
Electrochemical cell- It is a device which consists of two electrodes and an electrolyte to. Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells.
Electrochemical cells in which an externally supplied electric current is used to drive a chemical reaction which would not occur spontaneously are called electrolytic cells. All rights reserved. Classscience » Chemistry. Share with your friends.
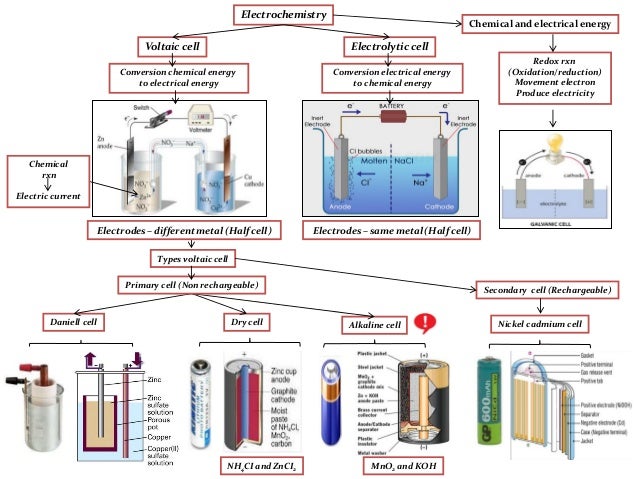
Ashish Khanna answered this. Dear Student Electrochemical cell- It is a device which consists of two electrodes and an electrolyte to i convert chemical energy into electrical energy ii convert electrical energy into chemical energy. Electrochemical cells which generate an electric current are called voltaic cells or galvanic cells Whereas Electrochemical cells in which an externally supplied electric current is used to drive a chemical reaction which would not occur spontaneously are called electrolytic cells.
View Full Answer. Aakash EduTech Pvt.]
I consider, that you commit an error. I can defend the position. Write to me in PM, we will discuss.