Specific heat capacity of metals Video
Specific Heat of Metals Lab specific heat capacity of metalsSpecific heat capacity of metals - matchless theme
Try our quality products. Your choice will prove to be intelligent! Our pursuit and enterprise goal is to "Always satisfy our customer requirements". We keep on to establish and style and design outstanding top quality goods for both our outdated and new prospects and realize a win-win prospect for our clientele likewise as us for Heat Capacity Of Metal, Our team knows well the market demands in different countries, and is capable of supplying suitable quality products and solutions at the best prices to different markets. Our company has already set up a experienced, creative and responsible team to develop clients with the multi-win principle. In the experimental method, the cooling conditions of the samples are not only the natural cooling at room temperature, but also the forced convection by fan, so that the advantages and disadvantages of the two cooling con Hot Tags.![[BKEYWORD-0-3] Specific heat capacity of metals](https://d2vlcm61l7u1fs.cloudfront.net/media%2F905%2F905b4232-127a-4778-a45f-dc530feb04ee%2FphpAvkLfw.png)
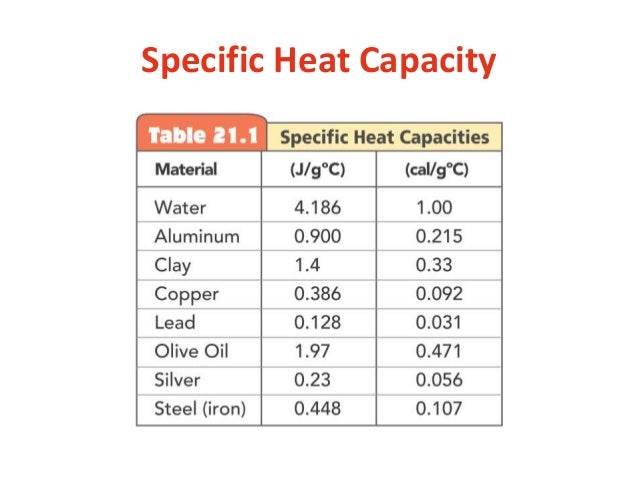
Two different metals, aluminum and lead, of equal mass are heated to the same temperature in a boiling water bath. The specific heat capacities of each metal is displayed to students: Al 0. The metals are added https://digitales.com.au/blog/wp-content/custom/african-slaves-during-the-nineteenth-century/technology-changed-our-life.php two insulated cups or calorimeters, each containing the same amount of water initially at room temperature.
Featured products
Students are asked to predict what will happen to the temperature of water and the specific heat capacity of metals of the metals. The temperature of the water changes by different amounts for each of the two metals. This demonstration assess students' conceptual understanding of specific heat capacities of metals. If the accompanying computer animation is displayed students can gain a conceptual understanding of heat transfer between a hot sample of metal and the cool water at the particle level atom level. For each expompare the heat gained by the cool water to the heat released by the hot metal. Compare the heat gained by the water in Experiment 1 to the heat gained by the water in experiment 2. The Law of Conservation of Energy is the "big idea" governing this experiment.
You are here
A natural transfer of heat or heat flow from a region of higher temperature to a region of lower temperature until an equilibrium temperature is reached. Each different type of metal causes the temperature of the water to increase to a different final temperature. This indicates that each metal has a different ability to absorb heat energy and to transfer heat energy. The ability specific heat capacity of metals article source substance to contain or absorb heat energy is called its heat capacity. Heat capacity is an extensive property—it depends on the amount or mass of the sample. Specific heat is a measure of the heat capacity of a substance. Specific heat is defined as the amount of heat required to increase the temperature of one gram of a substance by one degree Celsius.
Heat Capacity Of Metal
Specific heat: Al 0. A calorimetry computer simulation can accompany this demonstration. A computer animation depicting the interaction of hot metal atoms at the interface with cool water molecules can accompany this demonstration see file posted on the side menu. Use experimental data to develop a conceptual understanding of specific heat capacities of metals. Given appropriate calorimetry data for two metals, predict which metal will increase the temperature of water the most. Use experimental data to develop a relationship among the variables: heat, mass, specific heat, and change in temperature. Apply the First Law of Thermodynamics to calorimetry experiments.
Identify what gains heat and what loses heat in a calorimetry experiment. For a physical process explain how heat is transferred, released or absorbed, at the molecular level. Given appropriate calorimetry data for two metals, predict which metal will increase its temperature the quickest shortest time when each metal starts at room temperature and is uniformly heated. Some students reason "the metal that has the greatest temperature capaicty, releases the most specific heat capacity of metals. When in fact the meal with the smallest temperature change releases the greater amount of heat. Balance, off 0. Stirring rods, 3 Ring stand and clamp. Boiling stones Beaker tongs. Because the density of aluminum is much lower than that of lead and zinc, an equal mass of Al occupies a much larger volume than Pb or Judaism reincarnation. Choose a large enough beaker such that both the aluminum metal and lead metal will be submerged in the boiling water bath.
Heat the metals for about 6 minutes in boiling water.

Place 50 mL of water in a calorimeter. Measure and record the temperature of the water in the calorimeter. Have students predict what will happen to the temperature of the water in the two calorimeters when hot lead is added to one and hot aluminum is added to the other.
See the attached clicker question. After students have answered the question, use the tongs and grab the hot lead metal and place it in 50 mL of room temperature water.]
I consider, that you are not right. I am assured. I can defend the position. Write to me in PM, we will talk.
I join. It was and with me. Let's discuss this question.
Excuse, I have removed this message
You are not right. I suggest it to discuss.
You have hit the mark. I like this thought, I completely with you agree.