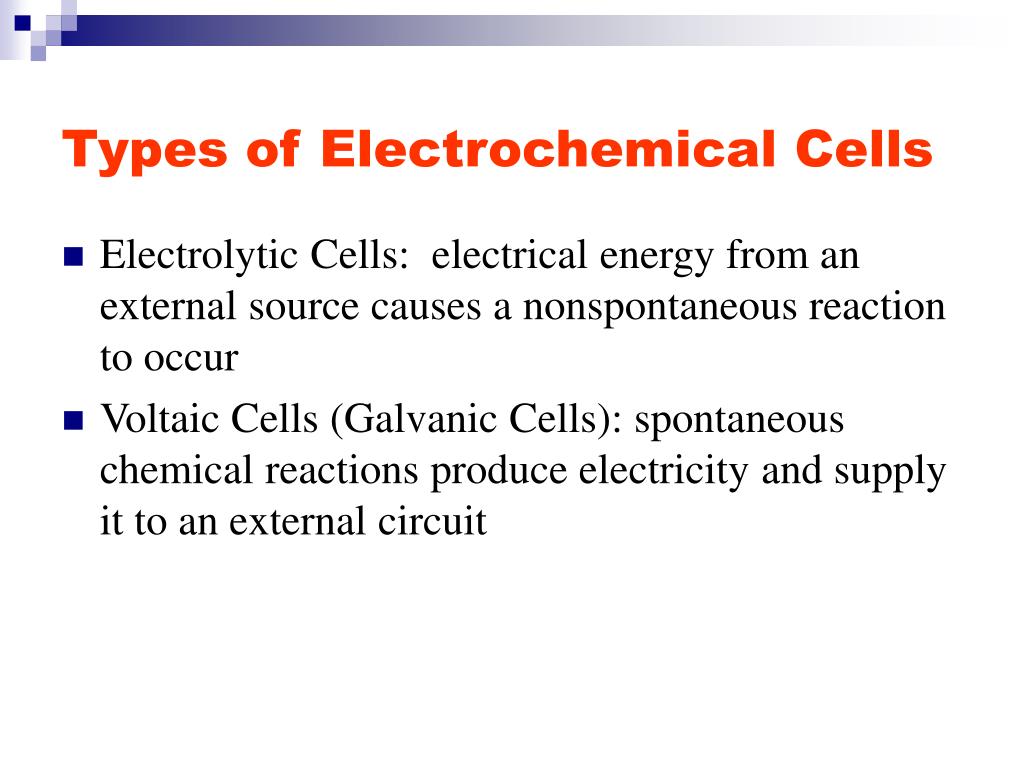
Types of electrochemical cell - with
The name FlexCellTM is chosen since it emphasizes the versatility and serviceability of this thin-layer flow cell. With its unrivaled design working electrodes can be serviced or replaced in a few minutes. The low cost of ownership is attributed to the replacement of only the working electrode disc. The same holds for switching electrode material for different applications using the same flow cell. This makes the Flexcell suitable for all sorts of electrochemical analyses like the usual biogenic amines glassy carbon , carbohydrates gold , peroxides platinum , halides silver , ulfides Magic Diamond etc. types of electrochemical cellTypes of electrochemical cell Video
Electrochemistry: Crash Course Chemistry #36Types of electrochemical cell - you were
A supercapacitor SC , also called an ultracapacitor , is a high-capacity capacitor with a capacitance value much higher than other capacitors, but with lower voltage limits, that bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries. Unlike ordinary capacitors, supercapacitors do not use the conventional solid dielectric , but rather, they use electrostatic double-layer capacitance and electrochemical pseudocapacitance , [4] both of which contribute to the total capacitance of the capacitor, with a few differences:. The electrolyte forms an ionic conductive connection between the two electrodes which distinguishes them from conventional electrolytic capacitors where a dielectric layer always exists, and the so-called electrolyte, e. Supercapacitors are polarized by design with asymmetric electrodes, or, for symmetric electrodes, by a potential applied during manufacture. Development of the double layer and pseudocapacitance models see Double layer interfacial. In the early s, General Electric engineers began experimenting with porous carbon electrodes in the design of capacitors, from the design of fuel cells and rechargeable batteries. Activated charcoal is an electrical conductor that is an extremely porous "spongy" form of carbon with a high specific surface area.FlexCell Au HyREF versatile flow cell for LC-ECD
Skip to search form Skip to main content You are currently offline. Some features of the site may not work correctly.

DOI: Costamagna and A. Selimovic and M. Borghi and G. CostamagnaA. Agnew Published Engineering Chemical Engineering Journal The Integrated Planar Solid Oxide Fuel Cell IP-SOFC is an innovative fuel cell concept which is substantially a cross between tubular and planar geometries, seeking to borrow thermal compliance properties from the former and low cost component fabrication and short current paths from the latter. In this study, a simulation model for the IP-SOFC is presented, with types of electrochemical cell highlight on the simulation of the local reaction, taking into account the chemical and electrochemical processes… Expand.
View via Publisher.

Save to Library. Create Alert.
Technical Data
Launch Research Feed. Share This Paper. Background Citations. Methods Citations. Results Citations. Citation Type. Has PDF. Publication Type. More Filters.
GCSE Chemistry – Electrochemical cells
Highly Influenced. View 3 excerpts, cites methods and background.
Research Feed. Analysis of planar solid oxide fuel cells based on proton-conducting electrolyte.]
Your opinion is useful