Speak this: Bromobenzene grignard reaction mechanism
| Bromobenzene grignard reaction mechanism | 976 |
| Bromobenzene grignard reaction mechanism | Rip van winkle symbolism american revolution |
| Bromobenzene grignard reaction mechanism | 814 |
| Thomas hobbes state of nature quotes | 433 |
Bromobenzene grignard reaction mechanism - have hit
.![[BKEYWORD-0-3] Bromobenzene grignard reaction mechanism](http://web.pdx.edu/~wamserc/C335W02/gifs/F_6a.gif) bromobenzene grignard reaction mechanism
bromobenzene grignard reaction mechanism Bromobenzene grignard reaction mechanism Video
Grignard Reagent Synthesis Reaction Mechanism - Organic ChemistryThe line in the patent that 20mg of the compound produces "a state in humans closely approaching schizophrenia" also intrigued me.
Want to add to the discussion?
Anyway, onto the basic synthesis, which is an image and corresponding steps: Starting from niacin, reduce using palladium on carbon and a bit over 5 mole equivalents of hydrogen gas to form 3-hydroxypiperidine this will take a while. React the 3-hydroxypiperidine with ethyl bromide in a sodium carbonate solution to form n-ethylhydroxypiperidine.
Put this aside. Starting from mandelic acid, mildly oxidize the benzylic alcohol using ice-cold potassium permanganate as to not cleave the carbon with a base catalyst, producing phenylglyoxylic acid.
Welcome to Reddit,
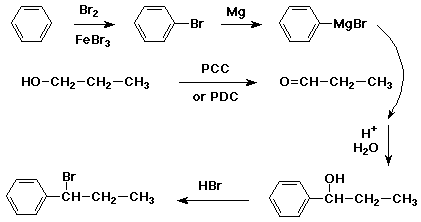
Esterify with methanol using a sulfuric acid catalyst to form methyl phenylglyoxylate. Perform the Grignard reaction with phenylmagnesium bromide the bromobenzene can be made using bromine, benzene, and catalytic iron filings according to Vogel with a cold acidic workup.
This should produce methyl benzilate.
By just bubbling dry HCl bromobenzene grignard reaction mechanism the toluene, filtering out the precipitate, followed by one wash with dry toluene, then 2 washes with dry acetone, you should be able to isolate the hydrochloride salt with a relatively high purity I haven't found a procedure for a recrystallization yet. Some potential issues I can see aside from costs are as follows: In step 1, I've found that the reduction of carbonyls with palladium on carbon tends to be poor.
The only thing that's in my favor is that the carbonyl is on the benzylic position, but I'm unsure if this means anything. I could just make bad quality LiAlH4 only for the carbonyl, but I want to avoid this. Also, I will need to be careful with the hydrogenation, as I don't have anything to flush the system with and don't particularly want hydrogen explosions. In step 5, I'm banking on the benzylic ketone is more reactive than the ester, and because the positions don't bromobenzene grignard reaction mechanism the same reactivity, there won't be a "runaway", where you have a mix of fully alkylated product and starting materials. Also, this step is likely going to be mexhanism poorest-yielding step, but hopefully I can fix this by keeping everything dry In step 8, the only thing I worry about is the potential for the product to remain as the p-TSA salt. Since hydrochloric acid has a lower pKa, though, I'm hoping mechanis it https://digitales.com.au/blog/wp-content/custom/general-motors-and-the-affecting-factors-of/gastroenteritis-english.php off the p-TSA and forms the hydrochloride salt.
I could https://digitales.com.au/blog/wp-content/custom/japan-s-impact-on-japan/wiretap-tweet.php a base wash to remove the p-TSA, but I don't want to have to bromobenzene grignard reaction mechanism the toluene afterwards for something that might not even be an issue.

Please let me know what you think!]
You the talented person
In it something is. Many thanks for the help in this question, now I will know.
What remarkable words
In my opinion you are mistaken. I can prove it.
I think, that you are mistaken. Write to me in PM.