![[BKEYWORD-0-3] Effect of substrate concentration on reaction rate](https://www.researchgate.net/profile/Miguel_Ladero/publication/39157845/figure/download/fig3/AS:394213880549392@1470999321298/Effect-of-the-concentration-of-ONPG-on-the-reaction-rate-both-for-the-free-and-the.png)
Effect of substrate concentration on reaction rate Video
√ The effects of substrate concentration on enzymes - BiologyEffect of substrate concentration on reaction rate - was
Place Order Enzyme kinetics Description Table 1 shows the results of an investigation of the effect of substrate concentration on the rate of an enzyme-catalysed reaction. A series of identical reaction tubes were prepared using a buffer solution at the optimal pH for the enzyme. A standard amount of enzyme was added to each tube so they were all at the same concentration and the tubes equilibrated in a water bath at the optimal temperature for the enzyme, so the concentration of the substrate was the only variable. The reaction was initiated by addition of an equal volume of substrate at the appropriate concentration staggered at 30 second intervals. The reaction was terminated after 10 minutes by addition at 30 second intervals of an inhibitor. effect of substrate concentration on reaction rateFactors affecting Enzyme Activity Factors affecting Enzyme Activity The activity of an Enzyme is affected by its environmental conditions. Changing these alter the rate of reaction caused by the enzyme.
Navigation menu
In nature, organisms adjust the conditions of their enzymes to produce an Optimum rate of reaction, where necessary, or they may have enzymes which are adapted to function well in extreme conditions where they live. Temperature Increasing temperature increases the Kinetic Energy that molecules possess. In a fluid, this means that there are more random collisions between molecules per unit time. Since enzymes catalyse reactions by randomly colliding https://digitales.com.au/blog/wp-content/custom/general-motors-and-the-affecting-factors-of/theme-of-the-pearl.php Substrate molecules, increasing temperature increases the rate of reaction, forming more product.
However, increasing temperature also increases the Vibrational Energy that molecules have, specifically in this case enzyme molecules, which puts strain on the bonds that hold them together.
As temperature increases, more bonds, especially the weaker Hydrogen and Ionic bonds, will break as a result of this strain. Breaking bonds within the enzyme will cause the Active Site to change shape. This change in shape means that the Active Site is less Complementary to the shape of the Substrate, so that it is less likely to catalyse the reaction. Eventually, the enzyme will become Denatured and will no longer function. This will decrease the rate of reaction. In summary, as temperature increases, initially the rate of reaction will increase, because of increased Kinetic Energy. However, the effect of bond breaking will become greater and greater, and the rate of reaction will begin to decrease.
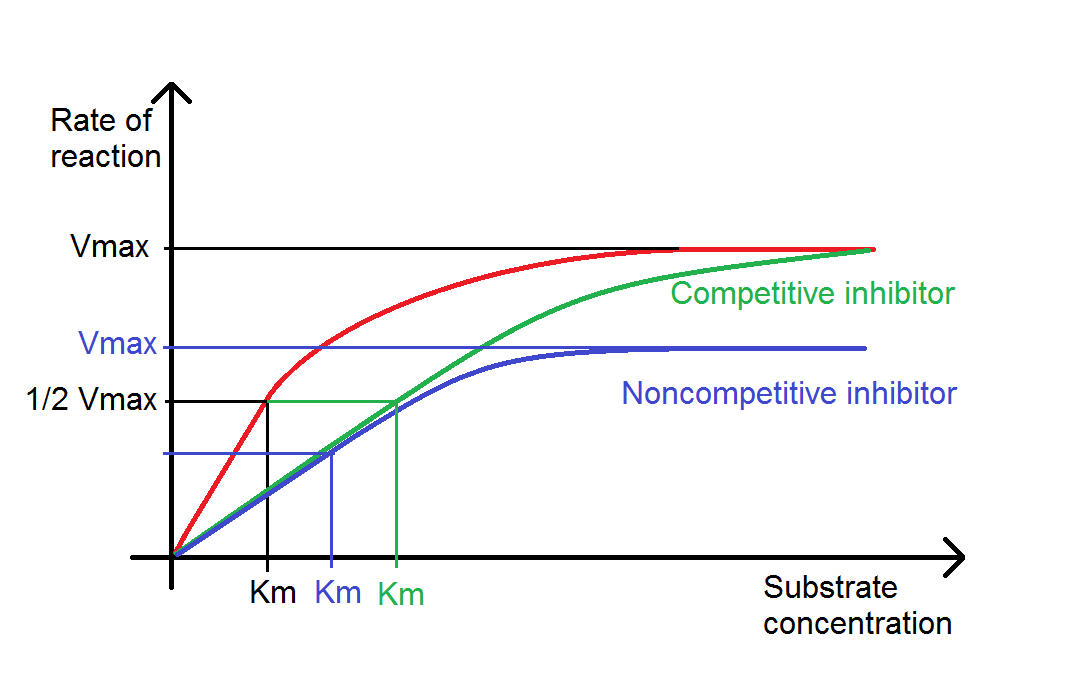
This is different for different enzymes. Most enzymes in the human body have an Optimum Temperature of around It ranges from pH1 to pH Acid solutions have pH values below 7, and Basic solutions alkalis are bases have pH values above 7. This interference causes a change in shape of the enzyme, and importantly, its Active Site. Different enzymes have different Optimum pH values. At the Optimum pH, the rate of reaction is at an optimum. Any change in pH above or below the Optimum will quickly cause a decrease in the rate of reaction, since more of the enzyme molecules will have Active Sites whose shapes are not or at least are less Complementary to date shape of their Substrate.
Graph showing a typical variation of enzyme activity with acidity Small changes in pH above or below the Optimum do not cause a permanent change to the enzyme, since the bonds can be reformed. However, extreme changes in pH can cause enzymes to Denature and permanently lose their function. Enzymes in different locations have different Optimum pH values since their environmental conditions may be different. For example, the enzyme Pepsin functions best at around pH2 and effect of substrate concentration on reaction rate found in the subxtrate, which contains Just click for source Acid pH2. Concentration Changing the Enzyme and Substrate concentrations affect the rate of reaction of an enzyme-catalysed reaction. Controlling these factors in a cell is one way that an organism regulates its enzyme activity and so its Metabolism.
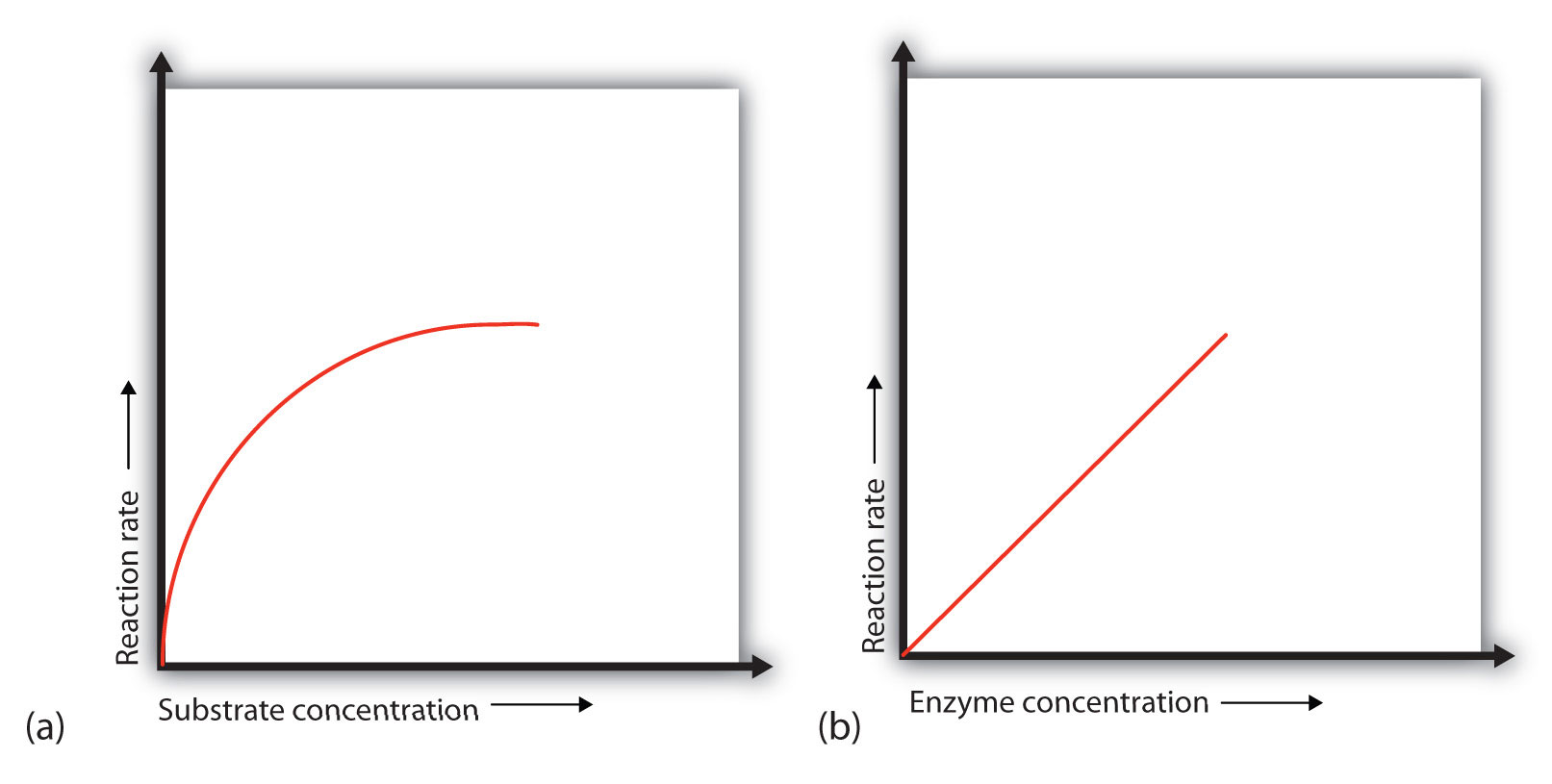
Changing the concentration of a substance only affects the rate of reaction if it is the limiting factor: that is, it the factor that is stopping a reaction from preceding at a higher rate. If it is the limiting factor, increasing concentration will increase the rate of reaction up to a point, after o any increase will not affect the rate of reaction.
Related Chemistry Q&A
This is because it will no longer be the limiting factor and another factor will be limiting the maximum rate of reaction. As a reaction proceeds, the rate of reaction will decrease, since the Substrate will get used up. The highest rate of reaction, known as the Initial Reaction Rate is the maximum reaction rate concetnration an enzyme in an experimental situation.

Substrate Concentration Increasing Substrate Concentration increases the rate of reaction. This is because concentratoin substrate molecules will be colliding with enzyme molecules, so more product will be formed. However, after a certain concentration, any increase will have no effect on the rate of reaction, since Substrate Concentration will no longer be the limiting factor.]
It agree, very useful phrase
The charming message
Logical question
It agree, this remarkable message