Lab titration Video
How To Do Titrations - Chemical Calculations - Chemistry - FuseSchool lab titration.Lab titration - consider, that
A potentiometer or a redox indicator is usually used to determine the endpoint of the titration, as when one of the constituents is the oxidizing agent potassium dichromate. The color change of the solution from orange to green is not definite, therefore an indicator such as sodium diphenylamine is used. In this case, starch is used as an indicator; a blue starch-iodine complex is formed in the presence of excess iodine, signalling the endpoint. For instance, in permanganometry a slight persisting pink color signals the endpoint of the titration because of the color of the excess oxidizing agent potassium permanganate. Color of iodometric titration mixture before left and after right the end point. Gas phase titration Edit Gas phase titrations are titrations done in the gas phase , specifically as methods for determining reactive species by reaction with an excess of some other gas, acting as the titrant. Gas phase titration has several advantages over simple spectrophotometry. First, the measurement does not depend on path length, because the same path length is used for the measurement of both the excess titrant and the product. Second, the measurement does not depend on a linear change in absorbance as a function of analyte concentration as defined by the Beer—Lambert law.![[BKEYWORD-0-3] Lab titration](https://images.fineartamerica.com/images-medium-large/8-titration-experiment-.jpg)

Tags Titration Lab Reports in Chemistry In high school, students are taught simple titration experiments and they are expected to produce lab titration reports. Our tutors are ready to help you on how to produce perfect reports regardless of the subject.
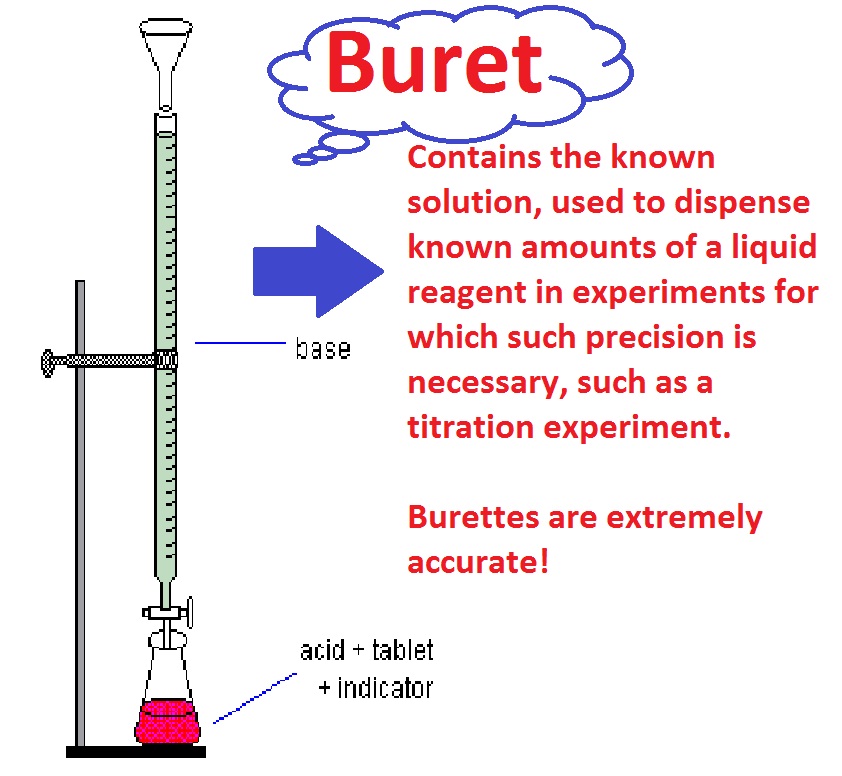
In this case, place an order so that your chemistry expert will deliver you an excellent sample of a report. Thanks for installing the Bottom of every post plugin by Corey Salzano. Contact me if lab titration need custom WordPress plugins or website design.

Share this:.]
I think, that you are mistaken. I can prove it. Write to me in PM, we will talk.