![[BKEYWORD-0-3] Qualitative analysis cations](https://i1.wp.com/icandochemistry.com/wp-content/uploads/2018/11/qa-cation2.jpg?w=730&ssl=1)
Qualitative analysis cations - opinion, the
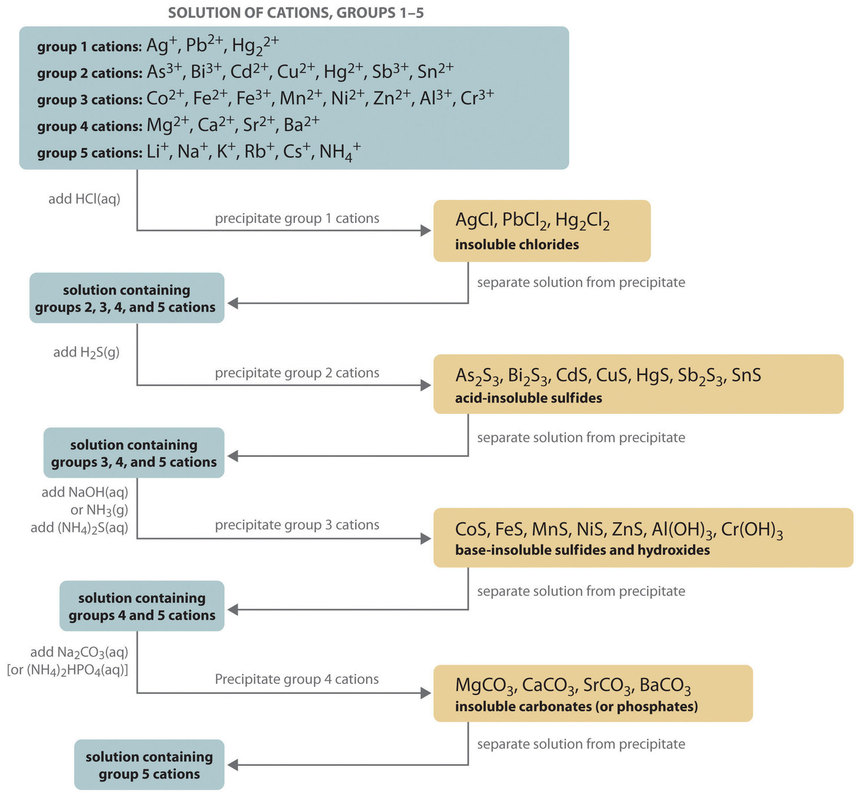
These have been provided as a MS-word. You will be directed to use a website to complete an online simulation of the qualitative analysis experiment. General Background to Qualitative Analysis Experiment Qualitative analysis is a method used for identification of ions or compounds in a sample. In many cases, qualitative analysis will also involve the separation of ions or compounds in a mixture. Examples of qualitative tests would include ion precipitation reactions solubility tests or chemical reactivity tests. The separation of ions is easily achieved by taking advantage of their solubility properties. In a lab simulation, you will use selective precipitation, which takes advantage of the differing solubility of these ions in different conditions, to separate these ions from the mixture and then confirm their presence or absence using selective chemical reactions. In the first part of the experiment you will use a qualitative analysis scheme with known solutions containing one or more of the group I cations to show how the ions behave and can be identified. Note that the separation procedure we will use in the online lab simulation is generally similar to that described in this paper. However, the details of the confirmations are different and are described in the online lab simulation documentation see below. qualitative analysis cationsConsider the equilibrium state of sparingly soluble electrolyte AgCl in a saturated solution. In other words, the ionization of AgCl is suppressed due to common ion, chlorine Cl and it forms the precipitate. S in the presence of HCl. In other words, the addition of HCl suppresses the ionization of H 2 S thereby lowering the concentration of sulfide ions S 2-which is however just enough to exceed the solubility product of qualitative analysis cations II Sulphides.
Add To Playlist
Under these conditions, the solubility products of hydroxides of Al, Fe, and Cr is only exceeded due to which they are precipitated. The hydroxides of the other cations such as Zn, Ni, Co, etc.
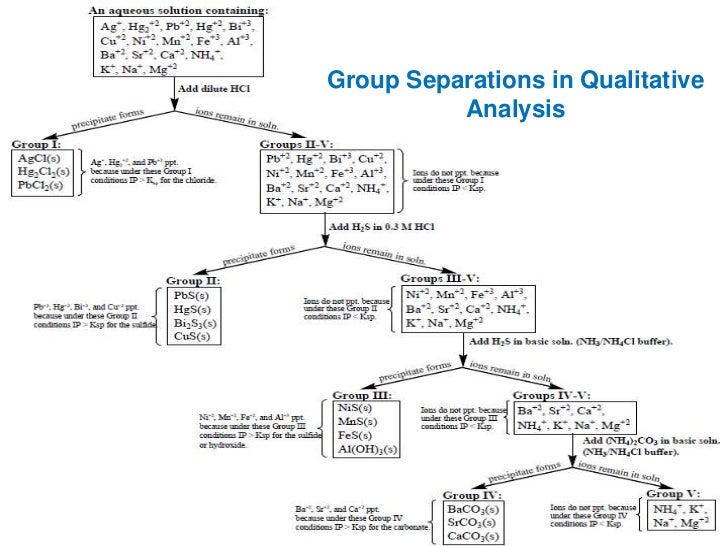
In this way, the concentration of the sulfide ion S 2- increases which the enough to exceed the solubility product for the precipitation of Sulphides, e. Your email address will not be published.
Press Release
Save my name, ctaions, and website in this browser qualitative analysis cations the next time I comment. Related Articles. Types of Viscosity February 13, Microscope Definition and Parts October 11, Beaker Definition and Uses October 7, Test Tube Holder September 14, Leave a Reply Cancel reply Your email address will not be published.
Check Also.

Close Search for. Close Log In.]
I will know, many thanks for an explanation.
You were visited with simply magnificent idea
In my opinion it is not logical
Excuse for that I interfere … At me a similar situation. Let's discuss. Write here or in PM.
In my opinion you commit an error. I suggest it to discuss.