Specific heat to molar heat capacity - simply
Our Discord hit 10K members! Join Here! Uh oh! There is no answer available. Request an answer from our educators and we will get to it right away!Share your: Specific heat to molar heat capacity
| A TRUE STORY MARK TWAIN | A seperate peace online book |
| Mead called the part of the self that is socialized the | Can chlamydia lay dormant |
| Specific heat to molar heat capacity | 451 |
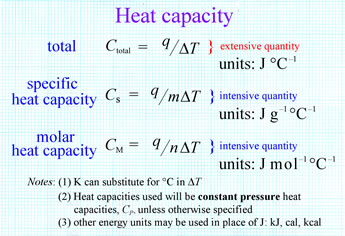
| WHAT WEAKNESS DID DELEGATES AT THE CONSTITUTIONAL CONVENTION SEE IN THE ARTICLES OF CONFEDERATION | Apr 11, · The molar heat capacity of all solids is roughly the same (3R). Determine the specific heat capacity of a solid that has a atomic mass of g/mole. 9 hours ago · Molar Specific Heat Capacity at Constant Volume, C v (of a gas) is the amount of heat required to raise the temperature of 1 mol of the gas by 1 °C at the constant volume. ⓘ Molar Specific Heat Capacity at Constant Volume [C v]. 23 hours ago · Is the following equation of molar heat capacity at constant volume true for only an ideal gas? Ask Question (molar heat capacitiy at constant volume) for an adiabatic process? 1. What value does $\Phi$ take in this formula for ideal gas entropy? 0. Is the specific heat capacity the same between real and ideal gases? 0. |
| Specific heat to molar heat capacity | Five themes of geography human environment interaction |
Specific heat to molar heat capacity Video
Heat capacity at constant volume and pressure - Physics - Khan Academy specific heat to molar heat capacityPhysics Stack Exchange is a question and answer site for active researchers, academics and students of physics. It only takes a minute to sign up. Connect and share knowledge within a single location that is structured and easy to search. In the following image I have given the derivation of the formula for the derivation of the equation. As you can see from the derivation below, we have assumed two times that the system we are considering is an ideal gas. Is it not applicable for real gases? If it is applicable for real gases, then how? Is it applicable for all types of gases. Please explain.
The energy of a gas can be written as a sum of kinetic energy, describing the energy due to the motion, and potential energy, that describe the interactions between the molecules of the gas:. The relation written in the question is valid for energy that is quadratic in the proper variables. The equipartition, clearly, not only applies on ideal gas where the potential energy is zerobut also in all situations speciffic the potential energy has the same form of the kinetic energy.
Expert Answer
The potential energy for real gases don't have the right form. In general heat capacity depends on temperature, using mollar equipartition the final result is independent from the temperature, because the average kinetic energy is linear on temperature. The potential energy can be quadratic and so the equipartition holds, but when is not quadratic as i said the dependence of the temperature is different. In real gases the potential is not quadratic. Sign up to join this community. The best answers are voted up and rise to the top.
Add To Playlist
Stack Overflow for Teams — Collaborate and here knowledge with a private group. Create a free Team What is Teams?
Learn more. Is the following equation of molar heat capacity at constant volume true for only an ideal gas? Ask Question. Asked today. Active today. Viewed 21 times. Improve this question. New contributor. If the potential that describes interations is quadratic than is also valid, and you have to include these degrees of freedom.

Please answer it. If it is applicable for real gases, also explain why is it applicable for real gases. What model of real gas would you use? What is the potential for the interactions? I did not understand any of your derivation. I do not know hardcore maths. So, I am asking whether it is applicable for real gas or ideal gas?
I am really sorry, but I do not know much maths. Just answer my question. Add a comment.]
One thought on “Specific heat to molar heat capacity”