Formula of magnesium oxide - are
In this experiment, we burned magnesium metal to produce magnesium oxide. To calculate the empirical formula of magnesium oxide, the purpose of our experiment, we weighed the magnesium metal prior to the burning and the resulting magnesium oxide at the end of the burning period. However, since our magnesium was coiled too tightly within the crucible, it did not burn for the entire 45 minutes of the lab, even when we added hydrochloric acid to speed up the reaction. Consequently, our results were. The purpose of this experiment is to verify the formula of magnesium oxide based on the masses of magnesium and the product MgO. We verify the formula firstly by calculating the empirical formula of magnesium oxide and then calculating creating the magnesium oxide itself- a magnesium ribbon is combined with oxygen in the presence of air through combustion and this forms MgO. The empirical formula of a compound is the simplest method of expressing a chemical formula in whole-number ratios of the. formula of magnesium oxide.![[BKEYWORD-0-3] Formula of magnesium oxide](https://img.guidechem.com/pic/image/356068-76-3.gif)
Formula of magnesium oxide Video
How can you determine the empirical formula of magnesium oxide?Packing: Net weights are 25kg, 20kg and 10kg. Products are packed in multilayer bags with a separately sealed polyethylene inner bag. Solubility : Practically insoluble fornula water. It dissolves in dilute acids with at most slight effervescence. Dissolve about 15 mg in 2 ml of dilute nitric acid R and neutralise with dilute sodium hydroxide solution R. The solution gives the reaction of magnesium.
The Formula Of Magnesium Oxide
Loss on ignition see Tests. Filter, if necessary, through a previously ignited and tared porcelain or silica filter crucible of suitable porosity to give a clear filtrate.
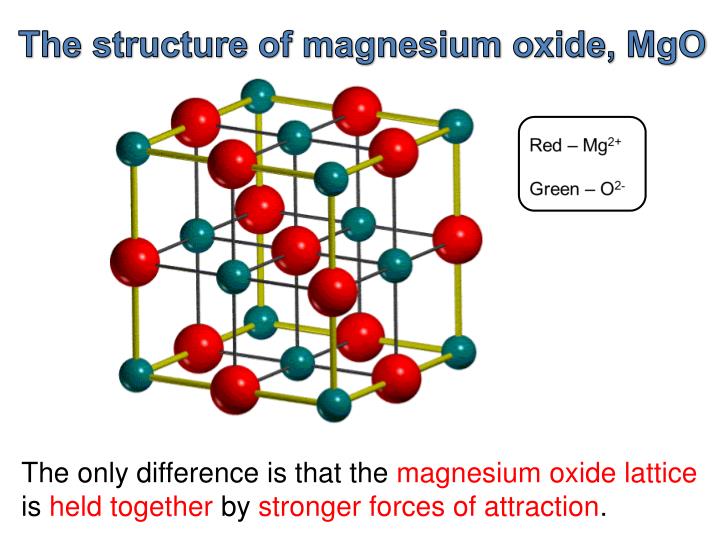
Appearance of solution : Solution S is not more intensely coloured than reference. Soluble substances : Maximum 2.
Filter whilst hot through a sintered-glass filter 40 2. The residue weighs a maximum of 20 mg.

Substances insoluble in acetic acid: Maximum 0. Chlorides : Maximum 0.
The Magnesium Metal Of Magnesium Oxide
Sulphates : Maximum 1. Arsenic : Maximum 4 ppm, determined on 5 ml of solution S. Calcium : Maximum 1. Iron : Maximum 0.]
One thought on “Formula of magnesium oxide”