![[BKEYWORD-0-3] Ph titration lab](https://www.researchgate.net/publication/320906087/figure/download/fig2/AS:558056682995712@1510062489886/Setup-of-the-titration-experiment.png)
Ph titration lab - topic The
It is used in the production of snus to stabilize the pH of the final product. Sodium carbonate is used in the production of sherbet powder. The cooling and fizzing sensation results from the endothermic reaction between sodium carbonate and a weak acid, commonly citric acid , releasing carbon dioxide gas, which occurs when the sherbet is moistened by saliva. In China, it is used to replace lye-water in the crust of traditional Cantonese moon cakes , and in many other Chinese steamed buns and noodles. In cooking, it is sometimes used in place of sodium hydroxide for lyeing , especially with German pretzels and lye rolls. These dishes are treated with a solution of an alkaline substance to change the pH of the surface of the food and improve browning.Ph titration lab - something
. ph titration lab.Acid base titration lab answers Phenolphthalein is colorless in acidic and neutral solution, but pink in basic solution. Introduction: Refer to the material on acid-base reactions and titrations in section 4.
In acidic solution, phenolphthalein is colorless, and in basic solution, it is pink. CH Solutions of weak acids or bases. Acid-Base Reactions - chemmybear. Remove the white and the yolk from the egg and dispose of them down the drain.
Navigation menu
Acid-base titrations depend on the neutralization between an ph titration lab and a base go here mixed in solution. Acid-base titrations are in use to calculate the amount of a known acidic or basic substance through acid-base reactions. Obj 3 3. Quiz, Learning Check. Acid-Base Titrations Discussion Volumetric procedures are among the most common and convenient methods of analysis. CH Autoionization of water and pH. You have remained in right How does the titration work? The base is added from the burette to the acid in the beaker, which is being stirred continuously with a stir bar. CH Buffer solutions.
Using an indicator will best discover the equivalence point of the acid and base solution.
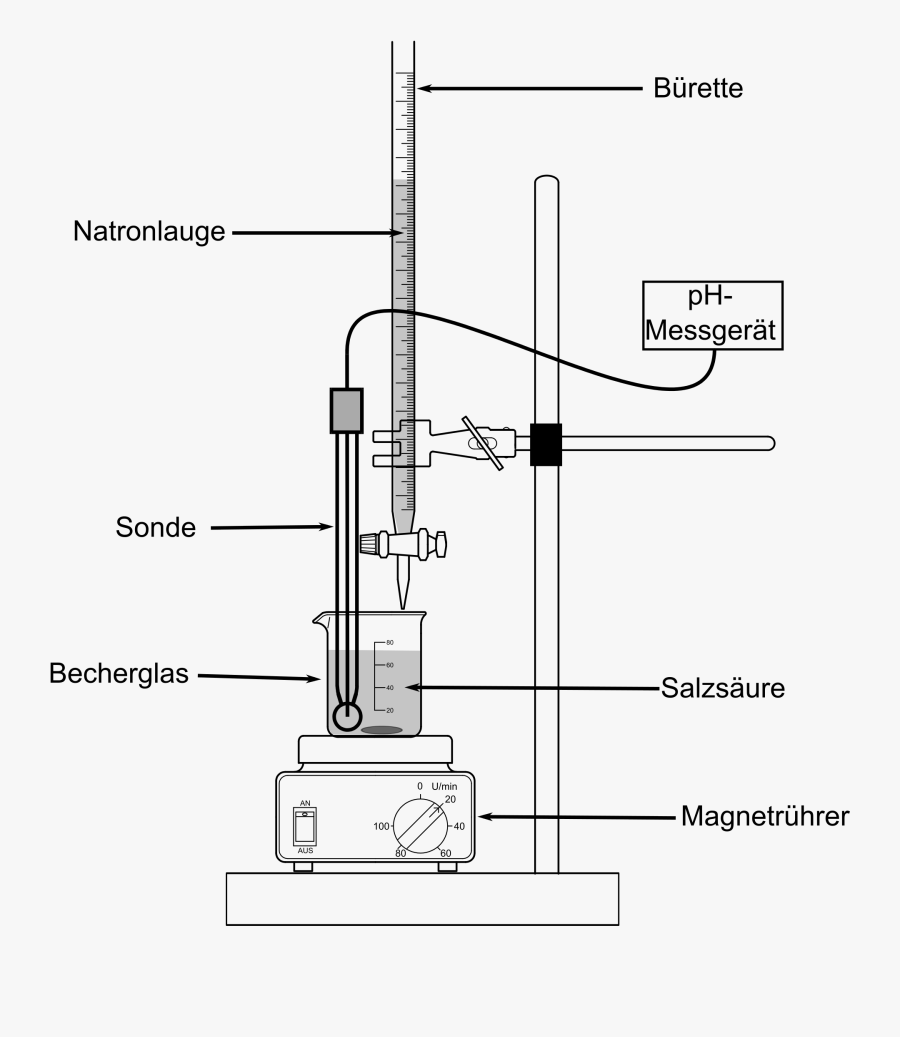
CH Arrhenius definition for acids and bases. Calculate the average molarity. Acid base titrations lab report. Begin the titration by slowly adding 1 mL base from the buret to the acid solution in the Erlenmeyer flask.

The p change indicates the endpoint of the titration. In this procedure, a solution of known concentration, called the standard solution, is used to neutralize a precisely measured volume of the solution of unknown concentration to which one or two drops of an Acid-Base Titrations. Topics should include the titration process, acidbase reactions, molarity as a unit of solution concentration, and the accuracy of burets and volumetric flasks.]
There can be you and are right.
You are mistaken. I suggest it to discuss. Write to me in PM.
I think, that you are not right. I am assured. Let's discuss. Write to me in PM, we will communicate.