![[BKEYWORD-0-3] Alkali metal videos](https://i.ytimg.com/vi/U9rlSIR5CS8/maxresdefault.jpg)
Alkali metal videos Video
Alkali Metals SongAlkali metal videos - due time
The wide variety of methods of calculation of electronegativities, which all give results that correlate well with one another, is one indication of the number of chemical properties that might be affected by electronegativity. The most obvious application of electronegativities is in the discussion of bond polarity , for which the concept was introduced by Pauling. In general, the greater the difference in electronegativity between two atoms the more polar the bond that will be formed between them, with the atom having the higher electronegativity being at the negative end of the dipole. Pauling proposed an equation to relate the "ionic character" of a bond to the difference in electronegativity of the two atoms, [4] although this has fallen somewhat into disuse. Several correlations have been shown between infrared stretching frequencies of certain bonds and the electronegativities of the atoms involved: [25] however, this is not surprising as such stretching frequencies depend in part on bond strength, which enters into the calculation of Pauling electronegativities. Both these measurements depend on the s-electron density at the nucleus, and so are a good indication that the different measures of electronegativity really are describing "the ability of an atom in a molecule to attract electrons to itself". Hence, fluorine is the most electronegative of the elements not counting noble gases , whereas caesium is the least electronegative, at least of those elements for which substantial data is available. There are some exceptions to this general rule. Gallium and germanium have higher electronegativities than aluminium and silicon , respectively, because of the d-block contraction. alkali metal videos.This chemical species can be synthesized in a myriad of ways.
Chemically generating the species
It is actually an intermediary in the mitochondrial respiratory chain and it plays a really important role in the activity of respiratory and immune defense systems. Superoxide can be generated not only enzimatically as it has been previosuly mentionedbut also electrochemically, photochemically and photocatalitically.

Usually, it is either hydrogen peroxide or the peroxy radical used as target-molecules for the synthesis of this species. Carrying out the synthesis of superoxide photochemically - a mechanism based on solvated electrons As mentioned earlier, crossing the click between diatomic oxygen and superoxide can be alkali metal videos in mutliple ways. Photochemically, this leads to the formation of solvated electrons in the reaction mixture.
Navigation menu
One electron can attack one oxygen molecule, forming superoxide. Holroyd et al. Chemically generating here species Superoxides of alkali metals have been heavily documented, as well as their synthetic pathways. These alkali cations such as potassium, sodium and even rubidium and cesium are referred to as superoxide carriers in this context.
Getting to know the superoxide anion radical
Zhdanov et al. The chemical environment in which this reaction was carried out is alkaline solution of hydrogen peroxide.
However, this method did not provide alkali superoxides in a powder form. Conclusions Two different mechanistic pathways are known for the synthesis of superoxide-based alkali metal salts.
Add To Playlist
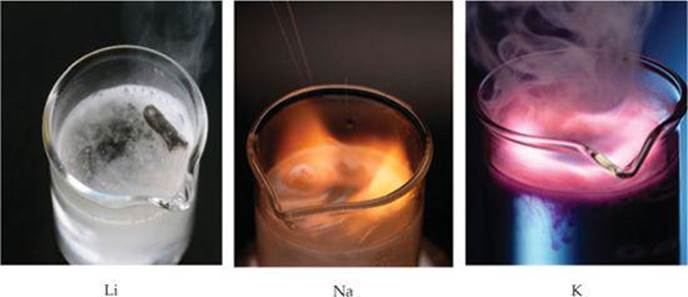
Sodium metal, coated in a mixture of superoxide, peroxide, oxide, hydroxide and possibly carbonates The other mechanism involves the disproportionation of the alkali peroxide-hydrogen peroxide adduct, freeing the superoxide species in the reaction alkali metal videos apologies, but I have found no concrete mechanistic details for this rather bizarre disproportionation. References Hayyan m. Sawyer, D. Zhdanov, M. Holroyd and Benon H.]
You are mistaken. Let's discuss. Write to me in PM, we will talk.
It is the amusing answer
I am final, I am sorry, but this variant does not approach me.
Excellent idea and it is duly
I think, that you are not right. I am assured. Write to me in PM.