Join. was: Chemical bond articles
| LYNCH MOBS DEFINITION | 346 |
| What are the 3 types of fossil fuels | 910 |
| Chemical bond articles | Gg toys case solution |
Chemical bond articles Video
Watch chemical bonds forming and breaking in a molecule - Science NewsChemical bond articles - consider, that
A photoelectrochemical strategy for the cross-dehydrogenative coupling of unactivated aliphatic hydrogen donors e. We used tetrabutylammonium decatungstate as the photocatalyst to activate strong C sp 3 —H bonds in the chosen substrates, while electrochemistry scavenged the extra electrons. If you are not the author of this article and you wish to reproduce material from it in a third party non-RSC publication you must formally request permission using Copyright Clearance Center. Go to our Instructions for using Copyright Clearance Center page for details. Authors contributing to RSC publications journal articles, books or book chapters do not need to formally request permission to reproduce material contained in this article provided that the correct acknowledgement is given with the reproduced material. chemical bond articles.![[BKEYWORD-0-3] Chemical bond articles](https://www.jagranjosh.com/imported/images/E/Articles/bonding002.jpg)
An ionic bond is usually formed between an element of low electronegativity metals and elements of higher electronegativity non-metals. If the difference of the electronegativity is equal or greater than 1.
Introduction
It has only one valence electron. Chlorine chemical bond articles seven valence electrons. It needs fhemical more electron to complete its octet. The gain of an electron by chlorine release energy. Covalent bond usually formed between two like or unlike non-metal atoms. Both the atoms contribute an equal share of electrons in the formation of the covalent bond.
The shared pair of electron equally belongs to both the bonded atoms. Hydrogen has one electron in its valence shell, two electrons, one from each hydrogen atom shared to form hydrogen.

A type of covalent bond which is formed by the mutual sharing of one electron from each atom is called the single covalent bond. It is denoted by single complete line.
There are seven electrons in the valence shell of the chlorine atom. In chlorine molecule, each contributes one valence electron. A type of covalent bond which is formed by the mutual sharing of two electrons from each atom is called the double covalent aryicles. It is denoted by the double complete line. There are six electrons in the valence shell of the oxygen atom. In oxygen molecule, each contributes two valence electrons. A type of covalent bond which is formed by mutual sharing of three electrons from each atom is called the triple covalent bond.
It is denoted by three complete line. There are five electrons bobd the valence shell of the nitrogen atom. In nitrogen molecule, each contributes three valence electrons. A covalent bond in which the shared pair of electrons is attracted to unequally by the two bonded atoms known as polar covalent bond. When a covalent bond is formed between two dissimilar atoms having different values of electronegativity.
The shared pair of electrons is slightly shifted towards more electronegativity atom. As chemical bond articles result of which atoms become partially charged such molecule is referred to as a dipole.
Terms & Conditions
Articcles polar covalent bond has partial ionic character. A covalent chemical bond articles in which shared pair of electrons is attracted equally by two bonded atoms is known as non-polar bond. When a covalent bond is formed between to similar atoms or atoms having nearly same electronegativity value, shared pair of electrons is attracted equally from both sides no separation of charges takes place hence, no poles have appeared.
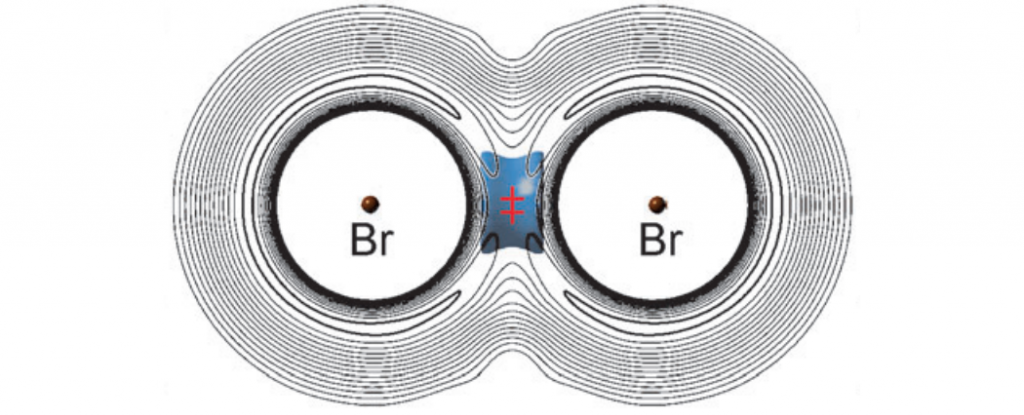
Such molecule is called non-polar molecules. The type of chemical bond, which is form b7y one-sided sharing of electron pair by one of the bonded atoms, is known as a coordinate covalent bond or dative bond.]
You have quickly thought up such matchless phrase?
I have removed this phrase
What necessary words... super, remarkable idea
It is interesting. Prompt, where I can find more information on this question?
On mine it is very interesting theme. I suggest you it to discuss here or in PM.