![[BKEYWORD-0-3] Latent heat of vaporization of nitrogen](http://www.atmo.arizona.edu/students/courselinks/fall10/nats101s11/lecture_notes/energy_temp_heat/LHvap_N2_expt.jpg)
Brilliant idea: Latent heat of vaporization of nitrogen
| Latent heat of vaporization of nitrogen | 2 hours ago · Water has a latent heat of fusion of J/g and a latent heat of vaporization of J/g. If grams of liquid water condenses, how much energy is involved in . Enthalpy / ˈ ɛ n θ əl p i / is a property of a thermodynamic system, defined as the sum of the system's internal energy and the product of its pressure and volume, H = U + pV. It is a convenient state function standardly used in many measurements in chemical, biological, and physical systems at a constant pressure. The pressure–volume term expresses the work required to establish the. 2 days ago · L+ NPM? (a) What is the enthalpy of reaction per gram of quicklime that reacts? (b) How much heat, in kilojoules, is associated with the production of 1 ton of slaked lime? KT Koketso T. Physics Electricity and Magnetism. 5 months, 1 week ago. A . |
| DOUMI CULTURE | Far from the madding crowd watch online |
| Cultural observation essay | 684 |
Latent heat of vaporization of nitrogen - happens
By Tony Markovich April 16, At a common gas pump, there are four, maybe five, different options presented to the buyer. Drivers can choose from regular unleaded with 87 octane, midgrade with 89 octane, premium with octane, diesel, and possibly E85 flex fuel with ethanol. At certain gas stations, however, there is another option: racing fuel. Regular gasoline and racing fuel cannot be used and discussed interchangeably because they have entirely different makeups. Not even every type of racing uses the same type of fuel, as each series and sport has vastly varied demands and requirements. In its simplest definition, racing fuel is a general classification for the type of fossil fuel used in and by racecars, race motorcycles, and other powersports vehicles in various professional and amateur racing series and events.What Is Racing Fuel?
For an exothermic reaction is more energy released in forming the bonds of the products or required in breaking the bonds of the reactants? If At a certain temperature, 3.
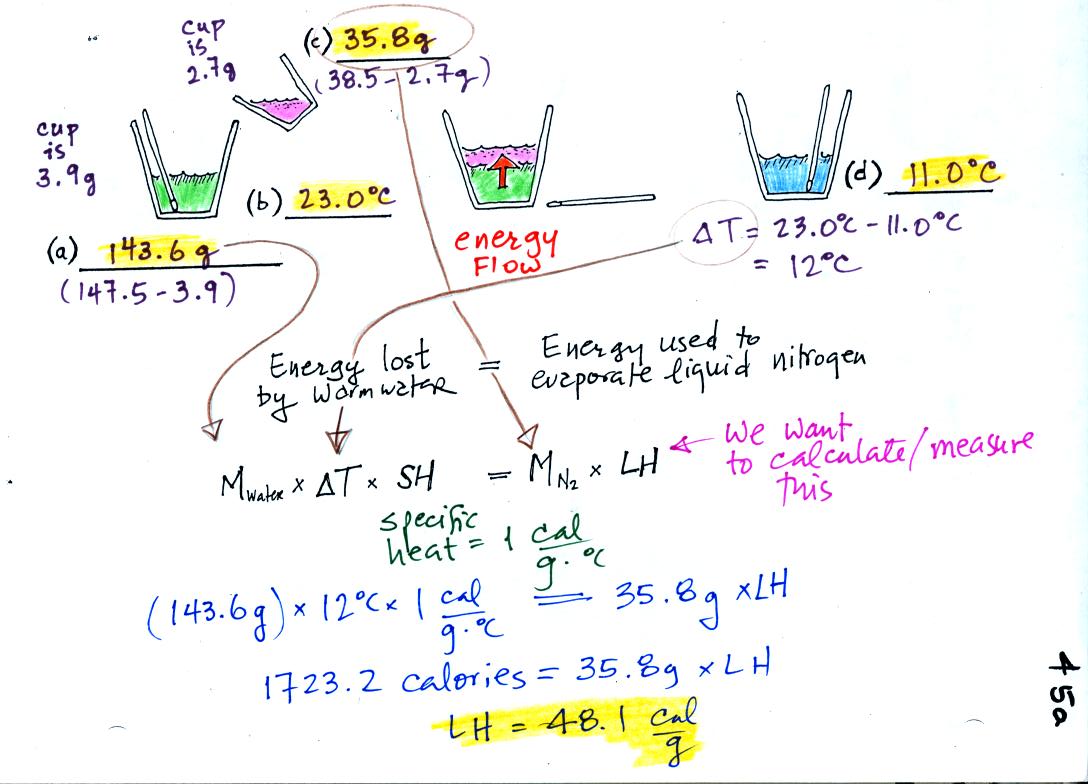
What is the temperature in Kelvin? How much energy was required to heat the metal if the specific heat of Al is 0. The pressure changes to 12 atm and the temperature changes to 2.
Why Do Different Racecars Use Different Racing Fuel?
This is the new volume of the balloon. Questions Responses.

Thermo: Ch Heat: Ch Redox: Ch Acid and Base: Ch Gas: Ch When HSO4 - acts as a base in a reaction, it becomes the chemical with the formula:. What is something at room temperature that would have a Standard Enthalpy of Formation of 0? What volume of 5.]
One thought on “Latent heat of vaporization of nitrogen”