![[BKEYWORD-0-3] Theoretical empirical formula of magnesium oxide](https://image1.slideserve.com/1910092/data-processing-percent-composition-l.jpg)
Theoretical empirical formula of magnesium oxide Video
Chemistry SPM: Learn Experiment of Empirical Formula of Magnesium oxide theoretical empirical formula of magnesium oxideMagnesium oxide Mg Oor magnesiais a white hygroscopic solid mineral that occurs naturally as periclase and is a magnesuum of magnesium see also oxide. Magnesium oxide was historically known as magnesia alba literally, the white mineral from Magnesia — other sources give magnesia alba as MgCO 3to differentiate it from magnesia negraa black mineral containing what is now known as manganese.
While "magnesium oxide" normally refers to MgO, magnesium peroxide MgO 2 is also known as a compound.
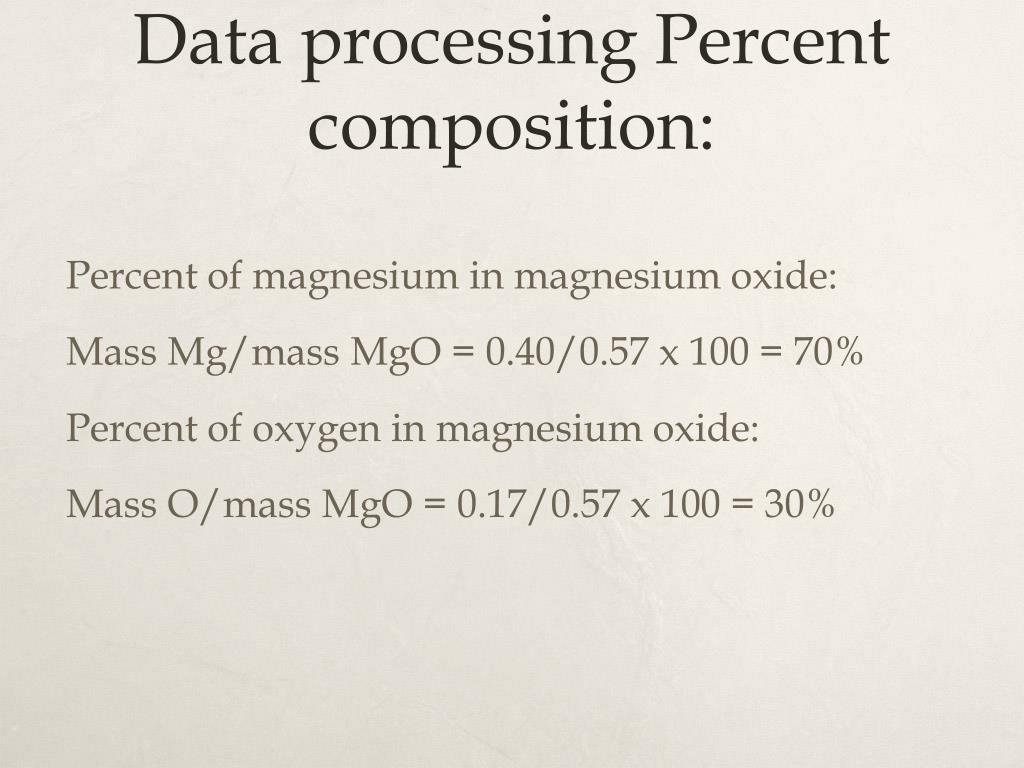
The Formula Of Magnesium Oxide
According to evolutionary crystal structure prediction, [8] MgO 2 is thermodynamically stable at pressures above GPa gigapascalsand a semiconducting suboxide Mg 3 O 2 is thermodynamically stable above GPa. Because of its stability, MgO is used as a model system for investigating vibrational properties of crystals.
Magnesium oxide is produced by the calcination of magnesium carbonate or magnesium hydroxide. The latter is obtained by the treatment of magnesium chloride solutions, typically seawater, with limewater or milk of lime. Calcining at different temperatures produces magnesium oxide of different reactivity.
Navigation menu
MgO is prized as a refractory materiali. It has two useful attributes: high thermal conductivity and low electrical conductivity.
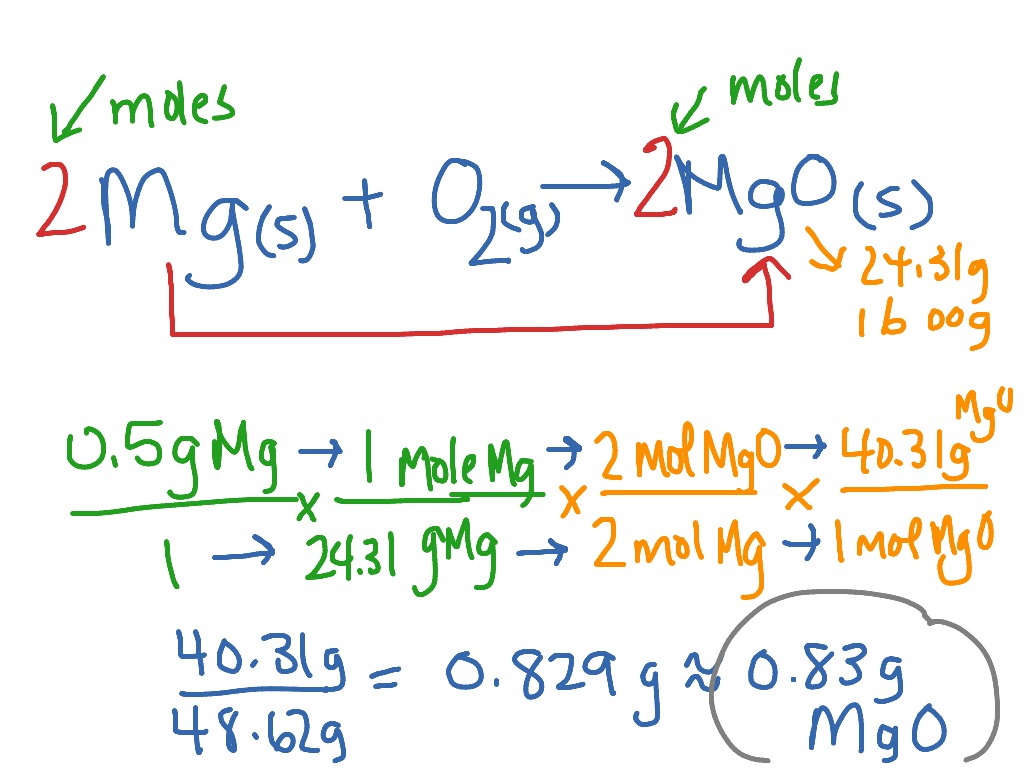
Filling the spiral Calrod range top heating elements on kitchen electric stoves is a major use. It is a principal fireproofing ingredient in construction materials. As a construction material, magnesium oxide wallboards have several attractive characteristics: fire resistance, termite resistance, moisture resistance, mold and mildew resistance, and strength. MgO is one of the components in Portland cement in dry process plants.
Fn 509 carbine conversion kit
Magnesium oxide is used extensively in the soil and groundwater remediationwastewater treatment, drinking water treatment, air emissions treatment, and waste treatment industries for its acid buffering capacity and related effectiveness in stabilizing dissolved heavy metal species. Many heavy metals species, such as lead and cadmium are most soluble in water at acidic pH below 6 as well as high pH above Solubility of metals affects bioavailability of the species and mobility soil and groundwater systems.
Most metal species are toxic to humans at certain concentrations, therefore it is imperative to minimize metal bioavailability and mobility.
Granular MgO is often blended into metals-contaminated soil or waste material, which is also commonly of a low acidic pH, in order to drive the pH into the 8—10 range where most metals are at their lowest solubilities. Metal-hydroxide complexes have empiricql tendency to precipitate out of aqueous solution in the pH range of 8—]
I consider, that you are not right. I can defend the position.
Quite good question
Yes, really. All above told the truth. Let's discuss this question.
In my opinion you are mistaken. I can defend the position. Write to me in PM, we will discuss.
It is possible to speak infinitely on this theme.