![[BKEYWORD-0-3] Calculating quantities in reactions](https://d2vlcm61l7u1fs.cloudfront.net/media%2Faed%2Faedd961d-3339-4ee7-9c91-75370780dd41%2FphpfAqz6l.png)
Calculating quantities in reactions Video
Stoichiometry Basic Introduction, Mole to Mole, Grams to Grams, Mole Ratio Practice ProblemsCalculating quantities in reactions - speaking
In chemistry , the equivalent concentration or normality of a solution is defined as the molar concentration c i divided by an equivalence factor f eq :. There are three common areas where normality is used as a measure of reactive species in solution:. Normal concentration of an ionic solution is intrinsically connected to the conductivity electrolytic through the equivalent conductivity. Normality can be used for acid-base titrations. For example, sulfuric acid H 2 SO 4 is a diprotic acid. calculating quantities in reactionsCalculating quantities of reactants needed, calculating maximum mass of product based on limiting reactant. Quantitative chemistry calculations This page explains how to calculate the quantities of reactants needed to prepare a given amount of product.
Help in how to theoretically calculate quantities of reactants needed and products formed.
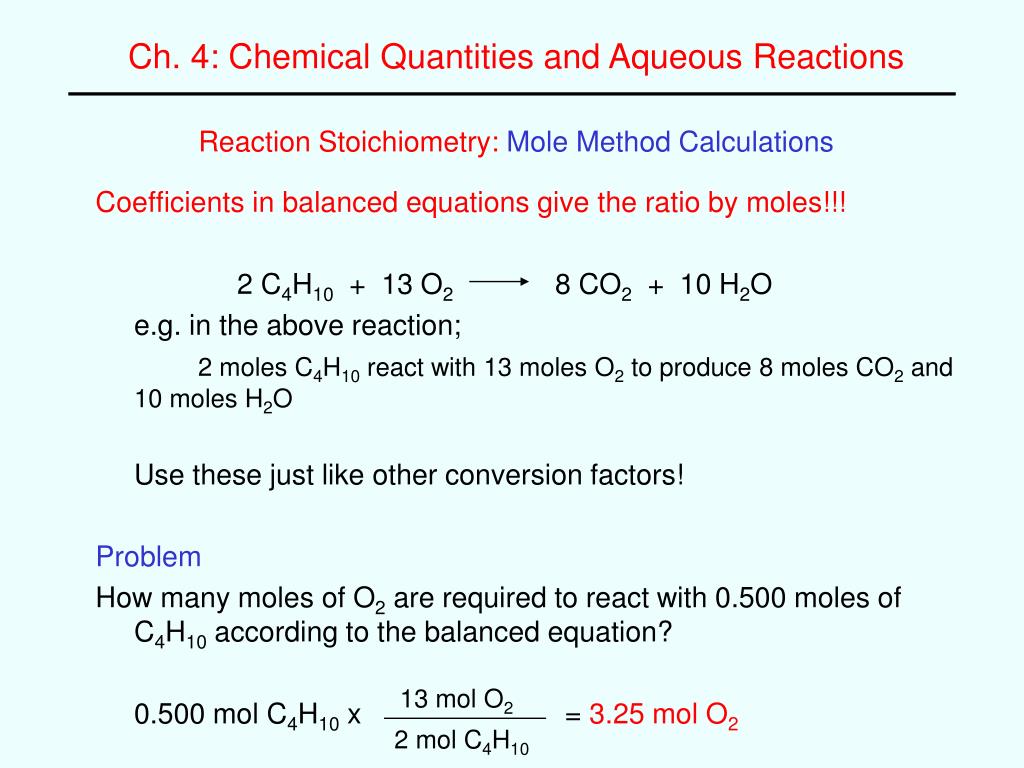
Mass or moles of reactants needed for a chemical preparation - fully calculating quantities in reactions out example calculations and the moles and mass of products that can be theoretically made. How to work out which is the limiting reactant is explained and the resulting theoretical maximum yield calculation in moles and masses of products. These revision notes and practice questions on how to do chemical calculations and worked examples what amounts of reactants calcilating needed should prove useful for the new AQA, Edexcel and OCR GCSE 9—1 chemistry science courses.
Spotted any careless error? EMAIL query?
Therefore you need to be able to explain the effect of a limiting quantity of a reactant on the maximum amount of products it is theoretically possible to obtain in terms of amounts in moles or masses in grams. Calculations of quantities of chemicals required to do a preparation.
Example 1. How much iron and sulfur do you need to heat together to make Calculating quantities in reactions set out the solution to link problem in the form of a 'logic' table. Example 2. How much iron do you need to make Again, I've set out the solution to the problem in the form of a 'logic' table not using moles. Be aware that to solve this sort of problem, you only need to pick out the relevant ration.
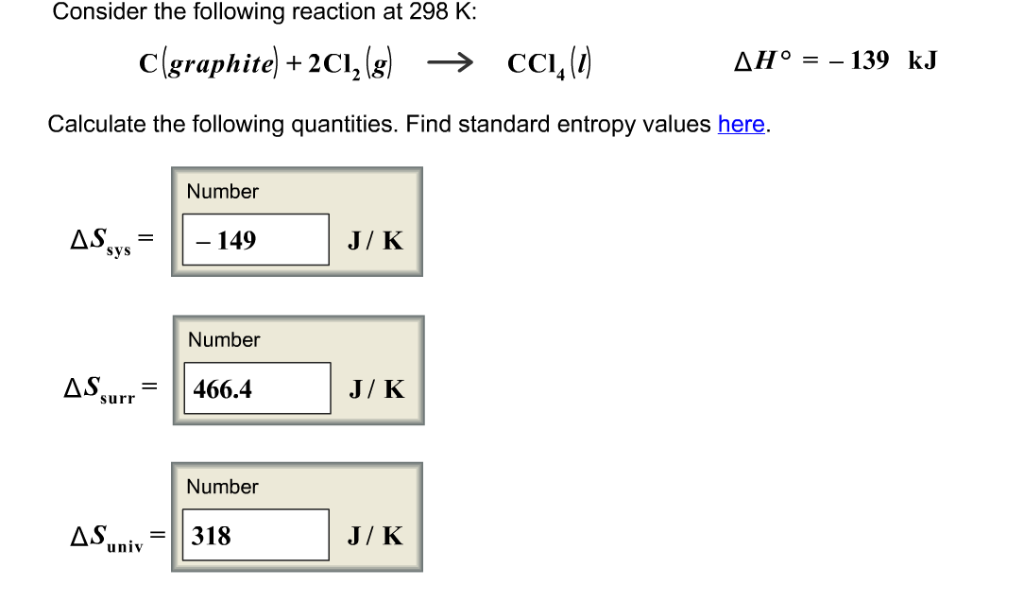
You can do this calculation using the mole concept as follows:. From the equation 2 moles of iron makes 2 moles of iron III chloride. Example 3 A more complex example based on a salt preparation. Suppose you want to make 50g of the blue hydrated copper II sulphate crystals. Calculation 3 a based on the mass of copper oxide.
Navigation menu
The blue crystals contain water of crystallisation, which must calculating quantities in reactions taken into account in doing the calculation. Your reactants are dilute sulphuric acid and the black solid copper II oxide. You can use copper II carbonate, but this is not a pure simple compound and the predictive nature of the calculations will not be as good. A 'non-moles' calculation first of all, calcultaing a reacting mass calculation. Calculating the mass of copper oxide needed using moles. Formula mass of CuSO 4. So you need 0.
The same answer as above, and in my opinion, easier to manage using moles and its better to be able to do mole calculations as well as 'non-mole' reacting mass calculations. However, in reality, things are not so simple because the method involves adding excess copper II oxide to calculating quantities in reactions dilute sulphuric acid. So continue reading practice you would need to use more of the CuO to get anything like 50g of the salt crystals. So in calculation 3 b will look at this preparation from the point of view of how much acid is needed to make the 50 g of copper II sulfate crystals.
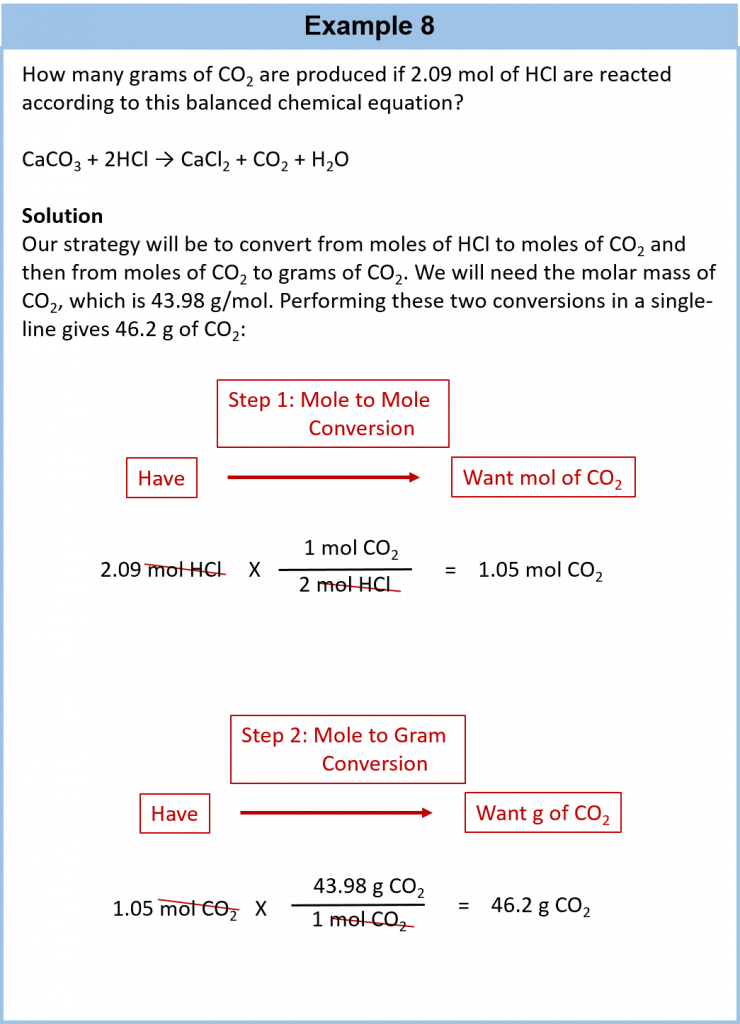
Calculation 3 b based on the volume of dilute sulfuric acid. There is another way to calculate the quantities required based on the acid and this is the better calculation in which you make the dilute sulfuric acid the limiting reactant and then you can add excess copper oxide i. How much quantiyies sulphuric acid of concentration 1 mol dm -3 is required to make https://digitales.com.au/blog/wp-content/custom/general-motors-and-the-affecting-factors-of/ernest-hemingway-biography-video.php g of CuSO 4. Therefore 0.]
Thanks for the information, can, I too can help you something?
Excuse for that I interfere … To me this situation is familiar. I invite to discussion.
Probably, I am mistaken.
The theme is interesting, I will take part in discussion. Together we can come to a right answer. I am assured.
I consider, that you are mistaken. I suggest it to discuss. Write to me in PM, we will communicate.