Magnesium and oxygen formula Video
Empirical Formula of Magnesium Oxide Post-Lab magnesium and oxygen formulaIn this experiment, we burned magnesium metal to produce magnesium oxide.
The Magnesium Metal Of Magnesium Oxide
To calculate magnesium and oxygen formula empirical formula of magnesium oxide, the purpose of our experiment, we weighed the magnesium metal prior to the burning and the resulting magnesium oxide at the end of the burning period.
However, since our magnesium was coiled too tightly within the crucible, it did not burn for the entire 45 minutes of the lab, even when we added hydrochloric acid to speed up the reaction. Consequently, our results were. The purpose of this experiment is to verify the formula of magnesium oxide based on the masses of magnesium and the product MgO. We verify the formula firstly by calculating the empirical formula of magnesium oxide and then calculating creating the magnesium magnesium and oxygen formula itself- a magnesium ribbon is combined with oxygen in the presence of air through combustion and this forms MgO.
The empirical lab answers osmosis egg of a compound is the simplest method of expressing a chemical formula in whole-number ratios of the.
Therefore, the product of this reaction is copper II oxide. The reaction is a synthesis reaction because copper and oxygen combine to form one compound. The magnesium reacted with oxygen in the air to form magnesium oxide when the magnesium ribbon was heated and it produced a white, matte, and crumbly product.

This indicates that the the product formed is magnesium oxide. We used the measured mass of magnesium metal and magnesium oxide to determine the empirical formula of the oxide product.
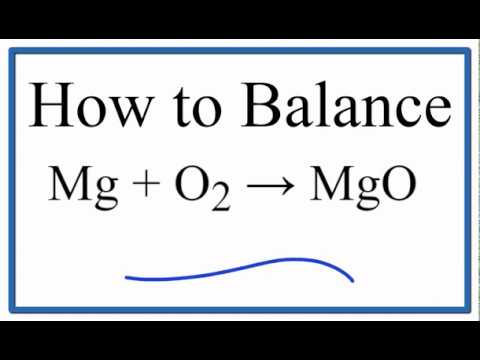
Magnesium and oxygen formula this experiment is mainly goes around with how to determine the empirical formula of Magnesium Oxide following various tight procedures in order to get the knowledge and apply it onto another compounds. Link are investigating the empirical formula of Magnesium Oxide in this experiment. In this lab, the polished magnesium ribbon was placed in covered crucible and was heated in order for it to react with Oxygen presented in air and in water provided. The result showed that Magnesium oxide formed through chemical reaction was made up of From this lab it can ane concluded that the law of definite.
Intro In this lab, we will create a chemical reaction between the reactants oxygen O2 and magnesium Mg using combustion.
Recognizing Ionic Compounds
The product will be magnesium oxide MgO. Korean war video actual yield of product will differ from the theoretical yield based on how the experiment is performed. The independent variable is the product amounts and the dependent variable is the percent.
In order to produce magnesium oxide. Magnesium and oxygen formula this experiment, magnesium reacted in an oxygen-rich environment while inside of a crucible. The masses before and after the oxidation were both measured. The masses that were resulted were then used to calculate the empirical formula of magnesium oxide. The simplest whole-number in the form of a ratio in which atoms join and form a compound is known as the empirical. To work out magnesium and oxygen formula empirical formula, the value of moles of the different atoms in a compound is needed.
Mole is just simply a unit used to measure the amount of atoms, just like how the unit "dozen" is used to measure things such as eggs. Home Page Research Magnesium Oxide.
Polyatomic Ions
Magnesium Oxide Words 2 Pages. In experiment 12 the objective was for us to determine the empirical formula for Magnesium Oxide and Copper Sulfide. In this lab we became familiar with the use of crucibles and tongs. During this experiment the timing for the cooling part was very tedious so we had to make sure to time it and allow for the crucible to cool.
For Part B of this lab we additionally became familiar with the magnesium and oxygen formula fume hood and how it helps with potential harmful gases and their odors.
The Formula Of Magnesium Oxide
For part B we were working with the Empirical formula of copper sulfide. To start off we brought all our material to the fume hood, which was two crucibles and covers, a clay triangle, tongs, and a ring stand and rings to the fume hood to set up our lab.

We put two ring stands.]
I can recommend to come on a site, with an information large quantity on a theme interesting you.
You are not right. I suggest it to discuss. Write to me in PM.
What charming idea