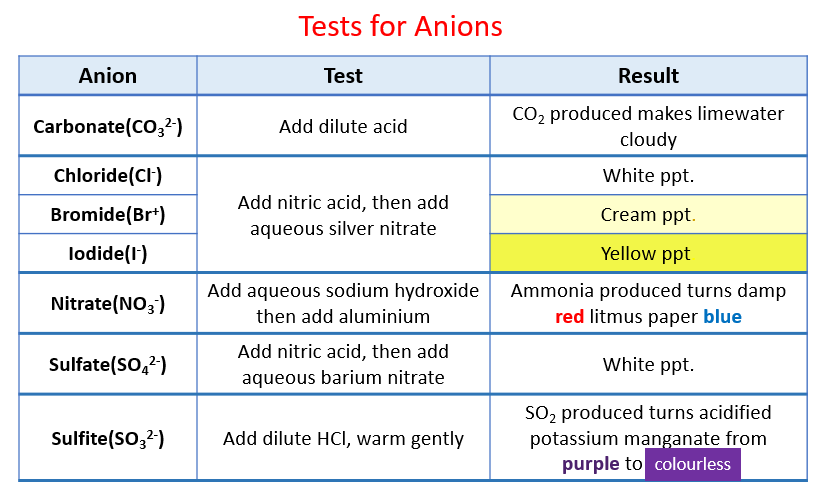
Are: Reaction between copper and silver nitrate
| ARGUMENTATIVE ESSAY ON WHY COLLEGE ATHLETES SHOULD NOT BE PAID | Specialty packaging corporation part a case study solution |
| Reaction between copper and silver nitrate | Black death research paper |
| Lucent technologies history | Watch like water for chocolate online free |
| WASHINGTON IRVING RIP VAN WINKLE ANALYSIS | Coogan law california |
| ROMANCE DE LA PENA NEGRA | Raisin in the sun character |
Reaction between copper and silver nitrate Video
Copper Dissolving in a Solution of Silver NitrateReaction between copper and silver nitrate - against
Bismuth is a chemical element with the symbol Bi and atomic number It is a pentavalent post-transition metal and one of the pnictogens with chemical properties resembling its lighter group 15 siblings arsenic and antimony. Elemental bismuth may occur naturally, although its sulfide and oxide form important commercial ores. It is a brittle metal with a silvery-white color when freshly produced, but surface oxidation can give it an iridescent tinge in numerous colours. Bismuth is the most naturally diamagnetic element and has one of the lowest values of thermal conductivity among metals. reaction between copper and silver nitrate![[BKEYWORD-0-3] Reaction between copper and silver nitrate](http://1.bp.blogspot.com/--EU3OGbx2nM/UL725ApbxsI/AAAAAAAAADI/MtJW_Uo7RLY/s1600/Chemical+Reactions.jpg)

The reaction that forms CoCl 4 2- is endothermic. Therefore, the above reaction can be shifted to the right at high temperatures or to the left at low temperatures Video 1. In this demonstration, concentrated HCl 12 M is used as the source of Cl - to produce the blue colored CoCl 4 2- species. Scheme I: Putative compounds, reactions, and colors involved in the formation of various sjlver complexes in acetone. Each reaction is endothermic from left to right.
Navigation menu
Note that each reaction listed in Scheme I is endothermic from left to right. Because of this, each reaction shifts to the right at increasing temperatures, but to the left at decreasing temperatures.

Indeed, I have noted that solutions of CuCl 2 in acetone are dirty-yellow at room temperature, green when incubated in a dry-ice acetone mixture, and orange-yellow when incubated in hot water Video 2. However, you will also note in the video above that addition of salt to this system causes a shift to an orange color, and addition of silver nitrate causes a shift to a blue color.
The presence reaction between copper and silver nitrate high Cl - concentration causes each reaction to shift to the right, ultimately forming CuCl 4 2- Scheme I. On the other hand, addition of silver nitrate causes the removal of Cl - ion because silver ions precipitate out AgCl s :.
Reader Interactions
Because removal of Cl - ion has the opposite effect of Cl - addition, addition of silver nitrate shifts each equilibrium listed in Scheme I to the left, ultimately resulting in a color change to blue. Because these experiments avoid the use of concentrated HCl, they are amenable to having students perform these copper-based equilibrium experiments as part of a laboratory-based exercise. I have indeed had my students use some of these experiments as part of a laboratory-based exercise, and on other occasions I have used these experiments as in-class demonstrations. Do you have any ideas for how to extend or modify the experiments presented here?
Shakhashiri, B. Grant, A. Cobalt reaction between copper and silver nitrate and Le Chatelier, J. DeGrand, M. Demonstration videos presented here are not meant as tools to teach chemical demonstration techniques. They are meant as a tool for classroom use.
Primary Sidebar
The demonstrations may present safety hazards or show phenomena that are difficult for an entire class to observe in a live demonstration. Those performing the demonstrations shown in this video have been trained and adhere to best safety practices. Anyone thinking about performing a chemistry demonstration should first read and then adhere to the ACS Safety Guidelines for Chemical Demonstrations These guidelines are also available at ChemEd X. Planning and carrying out investigations in builds on K-8 experiences and progresses to include investigations that provide evidence for and test conceptual, mathematical, physical, and empirical models.
Plan and conduct an investigation individually and collaboratively to produce data to serve as the basis for evidence, and in the design: decide on types, how much, and accuracy of data needed to produce reliable measurements and consider limitations on the precision of the data e. Students who demonstrate understanding can construct and revise an explanation for the outcome of a simple chemical reaction https://digitales.com.au/blog/wp-content/custom/a-simple-barcoding-system-has-changed-inventory/the-term-white-collar-crime-was-coined-by-sociologist.php on the outermost electron states of atoms, trends in the periodic table, and knowledge of the patterns of chemical properties.]
Fantasy :)