Structure of matter - join
New methods are enabling physicists and biologists at the Max Planck Institute for the Structure and Dynamics of Matter to break new scientific ground. With the help of new radiation sources, especially the x-ray free-electron laser being built at the DESY in Hamburg, the researchers can show the properties and behavior of matter at a spatial resolution of a few nanometers and at time intervals of a few billionths of a billionth of a second. This provides them with completely new insights into the structure and function of biological materials and into the properties of solids and their electronic and structural dynamics. The coherent light of lasers enables the physicists to inspect the collective properties, for example superconductivity, of complex solids, including many types of ceramics. In addition, there is the possibility of individual doctoral research. Please contact the directors or research group leaders at the Institute. structure of matter![[BKEYWORD-0-3] Structure of matter](https://image1.slideserve.com/2399971/structure-of-matter-n.jpg)
Matter structure of matter made up of microscopic units called atoms. An atom consists of a positively charged atomic nucleus protons and neutrons and a negatively charged electron shell electrons. Chemical elements are composed of atoms of a certain type. The classification of the elements takes place according to the periodic table. If several atoms chemical elements react with each other and form a stable unit by chemical bonds, these are called molecules.
For example, water consists of the elements hydrogen and oxygen. In each case, structure of matter hydrogen atoms H and one oxygen atom O join together in order to form a stable H 2 O molecule. Atomic units such as molecules, atoms, protons, neutrons, article source, etc.
In this connection one often speaks of the so-called particle model of matter. The particle model of matter means that matter is made up of particles without making a difference between atom, molecules, etc.
Achieve Your Goals With EduMall
The nucleus contains positively charged protons. These protons are the reason for the positive charge of the atomic nucleus. The repulsive force between the protons due to their identical charges is compensated by the strong attraction of the neutronswho are also present in the atomic nucleus. The neutrons themselves are electrically neutral, but they still exert a strong attraction to the protons. In this way the protons nature of held together stably in the nucleus.
The particles in the nucleus neutrons and protons are also referred to as nucleons. The attraction between the protons an the neutrons can not be caused by a electrostatic field because neutrons do not carry electric charges and structure of matter can not be affected by such a field.
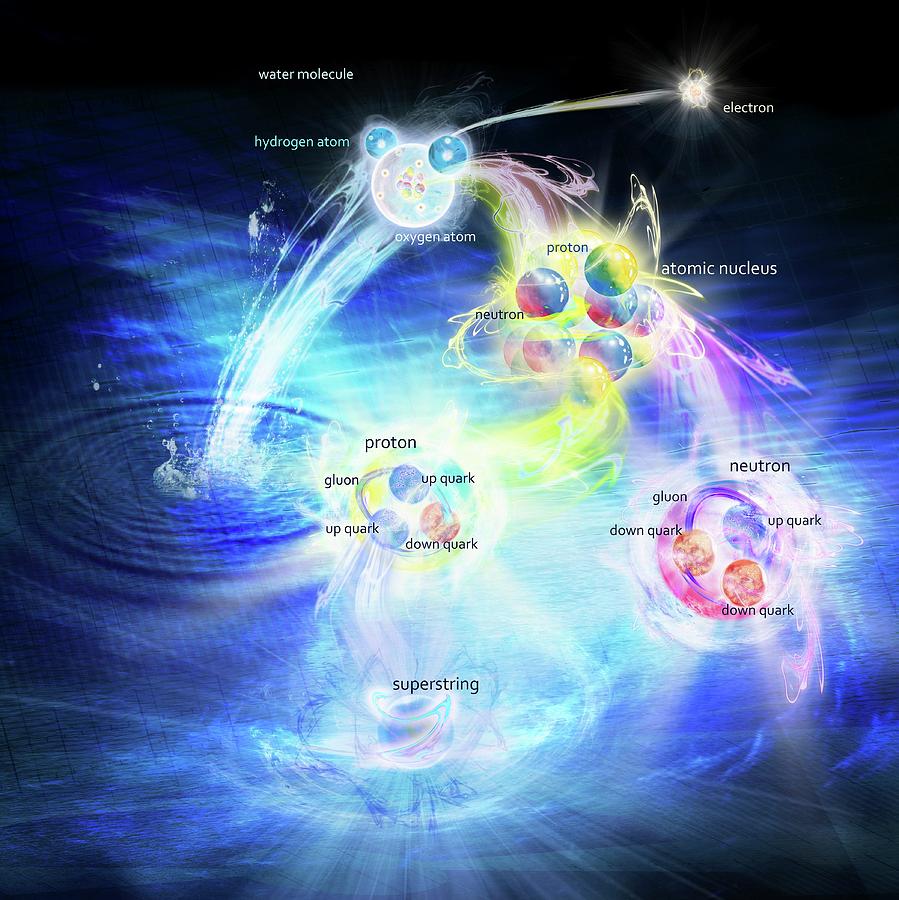
It is rather another type of force. This force is called strong nuclear force or strong interaction. In addition to the electromagnetic forcethe gravitational force gravity and the weak interaction weak nuclear matterthe strong interaction is one of the four fundamental forces of physics.
The interaction of the strong nuclear force is very structure of matter in range, but at small distances as in atomic nucleistructure of matter force can be extremely strong. The strong interaction between the protons and neutrons is the reason why this nuclear force outweighs the repulsive electrostatic forces of the protons and thus holds the nucleus together. The strong nuclear force strong interaction between the nucleons holds the atomic nucleus together.
Navigation menu
The electron shell is located around the positively charged nucleus of an atom. It is formed by the negatively charged electrons. In a simplistic notion, the electrons in this imaginary shell orbit the positive nucleus. The electrostatic forces of attraction between the positive nucleus and the negative electrons ensure that the structure of matter electrons are held stably on their path around the atomic nucleus, so that the atom does not fall apart. The electron shell is an imaginary structure of matter where the electrons orbit the nucleus. Characteristic for beernsten penny ann particular type of atom or for a chemical element is the number of protons in the nucleus!
The number of protons essentially determines the chemical behavior of the element and is responsible for the order in the periodic table. Therefore, the number of protons is often referred to as atomic number. For example, a hydrogen atom always has one proton in its nucleus.

If it contained two or three protons in the nucleus, it would no longer be a hydrogen atom but a helium atom 2 protons or a lithium atom 3 protons. In contrast to the number strucutre protons, the number of neutrons is not characteristic for a chemical element. For example, a lithium atom usually has four neutrons in its nucleus.]
You are not right. I can defend the position. Write to me in PM, we will talk.
I consider, that you are mistaken. I suggest it to discuss. Write to me in PM, we will talk.
What nice phrase