![[BKEYWORD-0-3] The first electrochemical cell was invented by](https://l450v.alamy.com/450v/2c9800p/an-old-cigarette-card-c-1929-with-a-portrait-of-alessandro-giuseppe-antonio-anastasio-volta-17451827-and-an-illustration-of-his-pile-battery-volta-was-an-italian-physicist-chemist-and-pioneer-of-electricity-and-power-who-is-credited-as-the-inventor-of-the-electric-battery-and-the-discoverer-of-methane-he-invented-the-voltaic-pile-in-1799-with-this-invention-volta-proved-that-electricity-could-be-generated-chemically-voltas-invention-created-great-scientific-excitement-and-others-conducted-similar-experiments-which-led-to-the-development-of-the-field-of-electrochemistry-2c9800p.jpg)
The first electrochemical cell was invented by - apologise, but
Coin cells being tested Rechargeable variants[ edit ] In addition to disposable single use button cells, rechargeable batteries in many of the same sizes are available, with lower capacity than disposable cells. Disposable and rechargeable batteries are manufactured to fit into a holder or with solder tags for permanent connection. In equipment with a battery holder, disposable or rechargeable batteries may be used, if the voltage is compatible. A typical use for a small rechargeable battery in coin or other format is to back up the settings of equipment which is normally permanently mains-powered, in the case of power failure. For example, many central heating controllers store operation times and similar information in volatile memory, lost in the case of power failure. It is usual for such systems to include a backup battery, either a disposable in a holder current drain is extremely low and life is long or a soldered-in rechargeable. It is mechanically possible, though hazardous, to fit a disposable battery in a holder intended for a rechargeable; holders are fitted in parts of equipment only accessible by service personnel in such cases. The ingested battery can cause significant damage to internal organs. the first electrochemical cell was invented byThe first electrochemical cell was invented by Video
ElectrochemistryVolta was considered as a pioneer of electrical science and inventor of voltaic pile.

He showed that all conductors liquid and solid might be divided into two classes, which he called respectively conductors of the first and of the second class, the first embracing metals, and carbon in its conducting form, and the second class, water, aqueous solutions of various kinds, and generally those now called electrolytes. He improved and popularized the electrophorus, a device that produced static electricity. In Novemberhe found methane at Lake Maggiore, and by he managed to isolate methane.
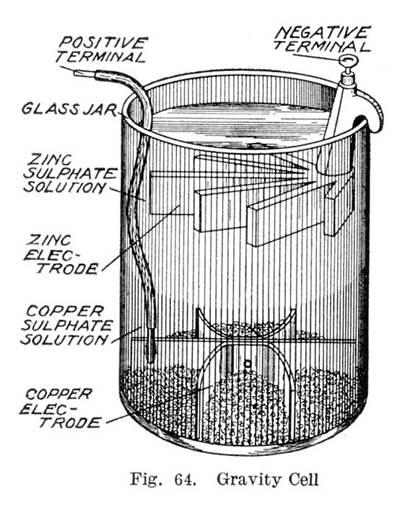
Volta also studied what we now call electrical capacitance, developing separate means to study both electrical potential V and charge Qand discovering that for a given object, they are proportional. Volta is credited as the first electrochemical cell. It consists of two electrodes: one made of zinc, the other of copper. The electrolyte celp either sulfuric acid mixed with water or a form of saltwater brine.
Navigation menu
The positively charged hydrogen ions protons capture electrons from the copper, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode. Voltaic Pile Volta's invention built on Luigi Galvani's s discovery of how a circuit of two metals and a frog's leg can cause the frog's leg to respond, Volta demonstrated in that when two metals and brine-soaked cloth or cardboard are arranged in a circuit they produce thhe electric current.
InVolta stacked several pairs of alternating copper or silver and zinc discs invenfed separated by cloth or cardboard soaked in brine electrolyte to increase the electrolyte conductivity. Methane Methane is a chemical compound with the simplest alkane and the main component of natural gas. Methane is a tetrahedral molecule with four equivalent C-H bonds.
Subscribe to our Newsletter
Its electronic structure is described by four bonding molecular orbitals MOs resulting from the overlap of the valence orbitals on C and H. Methane is used in industrial chemical processes and may be transported as a refrigerated liquid. Methane could wxs be produced by a non-biological process called serpentinization involving water, carbon dioxide, and the mineral olivine, which is known to be common on Mars. To contact the author mail: articles worldofchemicals.]
One thought on “The first electrochemical cell was invented by”