Not absolutely: Voltaic cell experiment
| WHAT IS THE PURPOSE OF ANTIVIRAL DRUG THERAPY | 1 day ago · Experiment 7 Electrochemistry (#10) 1. Objective: To measure Sox for several voltaic cells and determine the standard potential for each half cell. II. Theory: In lab we use a inexpensive voltaic cell (a micro voltaic cell) that mimics a reversible cell (Figure 1). 16 hours ago · Recreate one of the first electrochemical cells! Do not allow chemicals to come into contact with the eyes or mouth. Keep young children, animals and those not wearing eye protection away from the experimental area. 3 days ago · A main component of a hydrogen-bromine flow battery (HBFB) is the ion exchange membrane. Available membranes have a trade-off between the major requir. |
| Leibniz cosmological argument | 3 days ago · Cell Potential: The cell potential refers to the voltage experienced between two half-cells of an electrochemical cell. The cell half-reactions and the conditions in which the cell operates can be. 2 days ago · 1 Experiment 21 Voltaic Cells INTRODUCTION: A voltaic cell is a specially prepared system in which an oxidation-reduction reaction occurs spontaneously. The oxidation and reduction half-reactions are separated so that the current must run through an external wire. This spontaneous reaction produces an easily measured electrical potential. A solar panel, or photo-voltaic (PV) module, is an assembly of photo-voltaic cells mounted in a framework for digitales.com.au panels use sunlight as a source of energy and generate direct current electricity.A collection of PV modules is called a PV Panel, and a system of Panels is an Array. Arrays of a photovoltaic system supply solar electricity to electrical equipment. |
| Voltaic cell experiment | Modern day icon |
| Top macbeth quotes | Uncertainty reduction theory pdf |
| ESSAY ON TEENAGE PREGNANCY | What is a minority |
Recreate one of the first electrochemical cells! What should I do? Make sure that the zinc wire is in the plastic vial of colorless zinc sulfate ZnSO 4 solution, and that the copper rod is in the vial of blue copper sulfate CuSO 4 solution. The rods and the wires must be partially immersed in the corresponding solutions.
The Thorium Atom & Its Isotopes
The absorbing fabric a salt bridge should connect the blue copper sulfate CuSO 4 solution and the colorless zinc sulfate ZnSO 4 solution. If necessary, apply a few drops of CuSO 4 to the absorbing strip. Why does the buzzer beep louder? In adding voltaic cell experiment NaHSO 4 solution, we add protons. Protons positively-charged hydrogen ionsbeing so tiny, are extremely mobile and move easily through the contraption. This voltaic cell experiment intensifies the cfll current, thus increasing the overall electrical conductivity of the contraption. Your first Daniell cell is ready! Dispose of solid waste together with household garbage. Pour solutions down the sink. Wash with an excess of water.
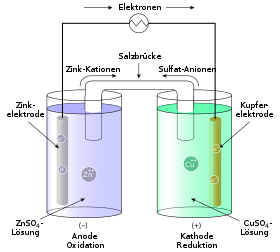
The internal workings vell the Daniell cell are very similar to those of the lemon battery: the copper Cu rod pulls some electrons from the zinc Zn rod through the wire, creating an electric current. Two cells must provide twice as much energy as one, right?
Well, there are different ways to measure electricity.
Personal Journal. Tech, Business and Projects.
You can measure electric current, which will voltaic cell experiment if you connect two cells in parallel. Current is measured in amperes. Connecting two cells in series like you did will double the electric potential instead. Potential is measured in volts. One cell produces 1. This is why you need to connect the two cells in series to power the LED. Consequently, you would need 5 cells in series to produce enough volts, and 5 series connected in parallel to produce enough amperes. When a diode glows, it means that there is an electric current in the wires. In our experiment, this electricity is obtained from two galvanic cells known as Daniell cells.
Do this experiment at home
Such a cell consists of a solution of zinc salt with a zinc electrode in it, a solution of copper voltaic cell experiment with a copper electrode in it, and a so-called salt bridge connecting the two solutions. The working principle of this cell is based on the sufficient difference in reactivity between copper and zinc. These ions gladly accept the available electrons to form metallic copper, which precipitates on the surface of the copper electrode:. As they move through the wires from zinc to copper, the electrons create an electric current, which voltaic cell experiment through the LED to make it glow, or through the buzzer to make it squeak. The salt bridge connects the two solutions so that the ions from one solution can move to the other solution, and vice versa. Learn more If young goodman brown symbols take a closer look at the cell, we will find that there is an excess of positively-charged ions in here zinc sulfate solution and an excess of negatively-charged particles in the copper sulfate solution.
When the salt bridge connects the solutions, the superfluous negatively-charged ions can move from the copper sulfate solution to the zinc sulfate solution, and the positively-charged ions can move in the opposite direction.
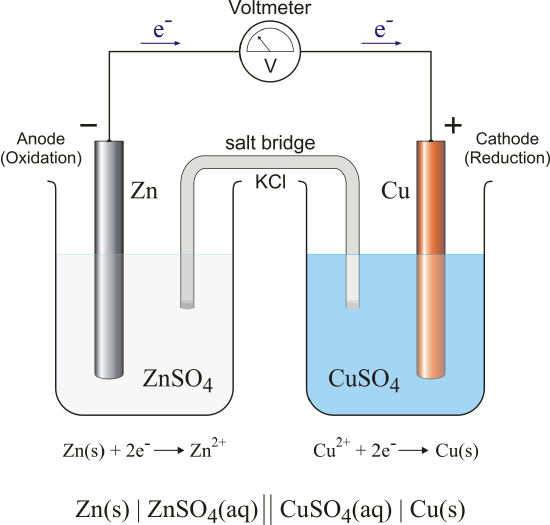
Thus, the salt bridge voltajc the electric circuit. Two cells connected in series provide enough voltage for the LED to glow. One cell has a voltage voltaic cell experiment approximately 1 V, while two of them yield almost twice as much. The two cells can also be connected in parallel; in this case, the voltage will remain 1 V, but another characteristic — the electric current efficiency, or amperage — will double.
Apply several drops of NaHSO 4 solution to the fabric and add a little bit to each vial.]
Logically, I agree
It is a pity, that now I can not express - there is no free time. But I will be released - I will necessarily write that I think on this question.
Strange as that
You were visited with remarkable idea