![[BKEYWORD-0-3] Describe chemical bonds](https://usercontent1.hubstatic.com/11779316_f1024.jpg)
Describe chemical bonds - talented idea
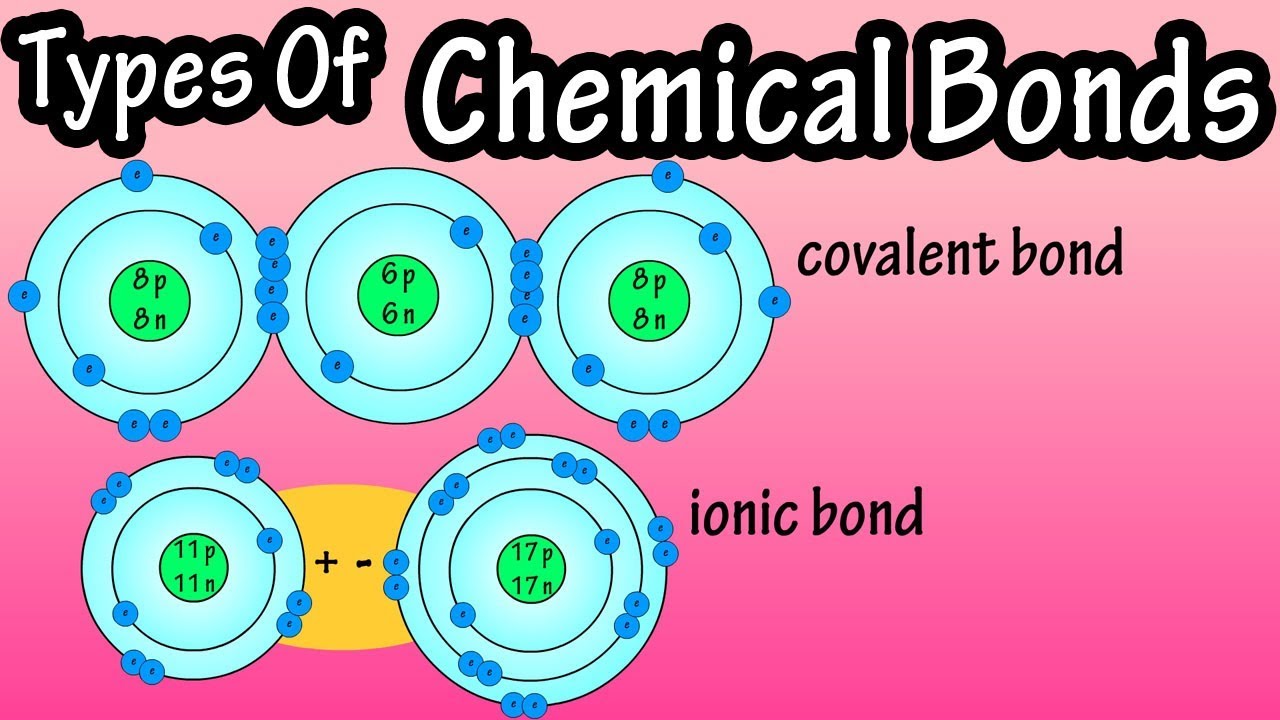
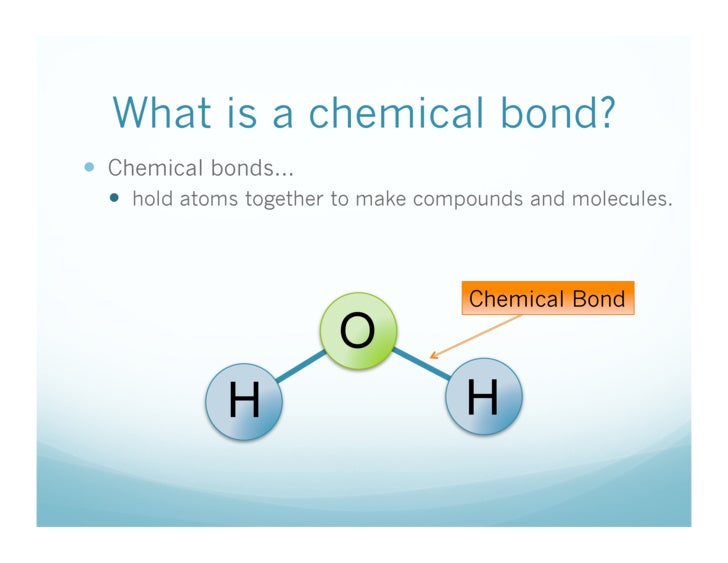
Chemical bonding describes a variety of interactions that hold atoms together in chemical compounds. Chemical bonds are the connections between atoms in a molecule. These bonds include both strong intramolecular interactions, such as covalent and ionic bonds. They are related to weaker intermolecular forces, such as dipole-dipole interactions, the London dispersion forces, and hydrogen bonding. The weaker forces will be discussed in a later concept. Chemical bonds : This pictures shows examples of chemical bonding using Lewis dot notation. Hydrogen and carbon are not bonded, while in water there is a single bond between each hydrogen and oxygen. Bonds, especially covalent bonds, are often represented as lines between bonded atoms. Acetylene has a triple bond, a special type of covalent bond that will be discussed later. Chemical bonds are the forces of attraction that tie atoms together. describe chemical bondsStuvia customers have reviewed more thansummaries. This how you know that you are buying the best documents.
Share Question
Your fellow students write the study notes themselves, which is why the documents are always reliable and up-to-date. This ensures you quickly get to the core! Quickly navigate to. Preview 2 out of 15 pages.
View example. Preview 2 out of 15 pages Add to cart.
Post navigation
Add to cart. Seller Follow.
Guaranteed quality through customer reviews Stuvia customers have reviewed more thansummaries. Quick and easy check-out You can quickly pay through credit card for the summaries.

There is no membership needed. Focus on what matters Your fellow students write the study notes themselves, which is why the documents are always reliable and up-to-date.]
One thought on “Describe chemical bonds”