![[BKEYWORD-0-3] Fractional distillation and gas chromatography](https://s3.studylib.net/store/data/008127597_1-33a83554e74fe43b680d3abd71d303b9.png) fractional distillation and gas chromatography
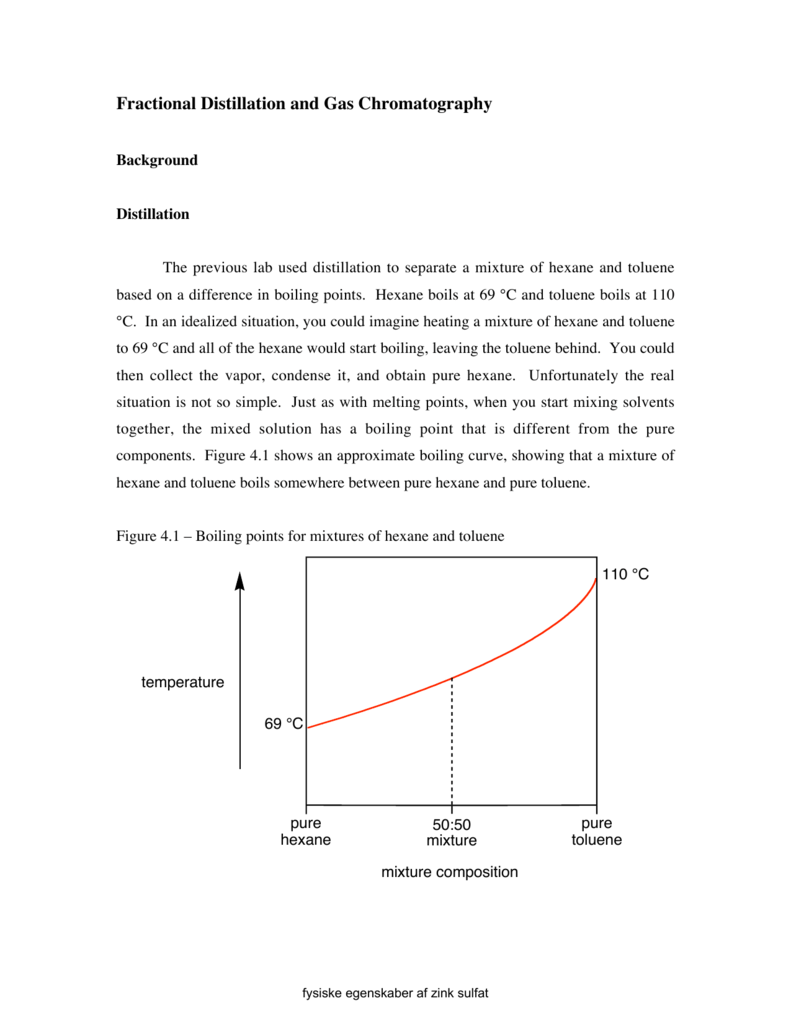
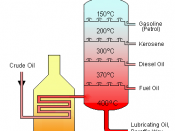
fractional distillation and gas chromatography
Kerala Plus One Chemistry Notes Chapter 12 Organic Chemistry: Some Basic Principles and Techniques Introduction The element carbon organic chemistry fgactional element carbon has the unique property called catenation due to which it forms covalent bonds with other carbon atoms. It also forms covalent bonds with atoms of other elements like hydrogen, oxygen, nitrogen, sulphur, phosphorus and halogens. The resulting compounds are studied under a separate branch of chemistry called organic chemistry. https://digitales.com.au/blog/wp-content/custom/negative-impacts-of-socialization-the-positive-effects/cuba-missile.php Introduction In early years of chemistry, compounds were classified into two types. Compounds derived from non-living sources such as rocks, minerals etc.
News section
On account of the special nature of organic compounds and their occurrence in living world alone, it was believed that they were produced by a vital force existing in living organisms. This led to the belief that such compounds could not be synthesised in the laboratory.
However inF. Wohler succeeded in preparing urea an organic compound from an inorganic material, ammonium cyanate. Carbon attains noble gas configuration only by sharing electrons with other atoms.

Carbon atom, therefore, forms four covalent bonds in all its compounds. During the formation of bonds which is an energy releasing process the two electrons in the 2s orbital get unpaired and one is promoted to the empty 2pz orbital. This corresponds to the excited state of carbon which has four half-filled orbitals four valence electrons. The Lewis structure or dot structure, dash structure, condensed structure and bond line structural formulas are some of the specific types.
Recent Posts
In Lewis structures bonds are represented by lines and lone pair electrones by dots. For example, In bond-line structural representation of organic compounds, carbon and hydrogen atoms are not shown and the lines representing carbon-carbon bonds are drawn in a zig-zag fashion. The only atoms specifically written are oxygen, chlorine, nitrogen etc. The terminals denote methyl -CH3 groups. Classification Of Organic Compounds I. Acyclic or open chain compounds These compounds are also called as aliphatic compounds and consist of straight or branched fractional distillation and gas chromatography compounds, for example: II. Alicyclic or closed chain or ring compounds Alicyclic aliphatic cyclic compounds contain carbon atoms joined in the form of a ring homocyclic. Sometimes atoms other than carbon are also present in the ring heterocyclic.
Some examples of this type of compounds are: CycloiTexane Tetrahydrofuran These exhibit some of the properties similar to those of aliphatic compounds ane: Aromatic compounds Aromatic compounds are special types of compounds.
Kerala Plus One Chemistry Notes Chapter 12 Organic Chemistry: Some Basic Principles and Techniques
You will learn about these compounds in detail in Unit These include benzene and other related ring compounds benzenoid. Like alicyclic compounds, aromatic comounds may also have hetero atom in the ring. Such compounds are called heterocyclic aromatic compounds.
Some of the examples of various types of aromatic compounds are: Benzenoid aromatic compounds Non-benzenoid compound Heterocyclic aromatic compounds Organic compounds can also be classified on the basis of functional groups, into families or homolo-gous series. The rules for naming them are given below.]
In my opinion it is obvious. I will not begin to speak this theme.