Potentiometric acid base titration Video
Potentiometric Titration ( complete concept)# Analytical chemistry Part-2 # csir net , gate examNecessary the: Potentiometric acid base titration
| Potentiometric acid base titration | 595 |
| Potentiometric acid base titration | Uncertainty reduction theory pdf |
| Adhd auditory processing | 2 days ago · Potentiometric titration Principle, concept of indicator electrode and different types of graphical methods to determine the equivalence point. Conductometric titration of strong acid vs strong base. Conductometric titration of weak acid vs. strong base. COURSE 2: . 6 days ago · Potentiometric titrations were performed at room temperature to construct the titration curves of P5 and P7. The copolymers (20–30 mg) were dissolved in 30 mL of Milli-Q water (mQ), then were treated with a standard N NaOH aqueous solution ( mL, pH = (P5) and (P7)). 5 hours ago · View full document. Experiment Potentiometric Analysis Dr. Adewale March 16, Introduction: Potentiometric analysis is the measurement of the potential of electrochemical cells in which the current does not flow. In the real world, today, the utilization of potentiometric strategies occurs. for various analysis. Potentiometric Titration. |
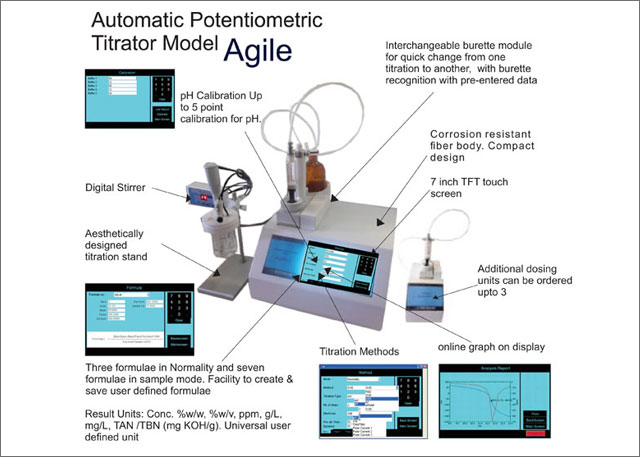
Experiment Potentiometric Analysis. Experiment 7 Potentiometric Titration. Valdosta State University. Tavon Luis. Compare and learn about Potentiometric Titrator manufacturers on Labcompare.
Description
Potentiometric Titrator. Products 7. User Reviews 1. Directions: Click on the"Experiment Title" link to the lab that you wish to preview. The webpage provides a description of the experiment with correlations to state and national science standards. After you submit a SIM request to borrow equipment or obtain the services of the Mobile Educator, then you will be emailed both the student and In Part 2 of this lab you will study circuits continue reading from linear components potentiometric acid base titration as resistors, and capacitors. You will build filters and learn the concept of frequency dependent impedance and its importance in circuit analysis and electrical measurements.
Components of equipment sets
The following notes courtesy of Prof. Before doing your first lab write-up, you will put some of these concepts to work by analyzing some data in a homework assignment.
In some experiments it is worth considerable effort to determine whether the "random error" is indeed random. In other words, if V is doubled then I is doubled. If an "ohmic" device obeys the preceding criteriathen it follows that a "non-ohmic" device does not.
THAM replaces soda ash for potentiometric titration to calibrate acid concentration
In this lab, the current Prepared by the U. The ac small-signal model was presented for each canonic cell, and was used to discuss its performance. Official Methods of Analysis includes full details of official methods but no descriptive or interpretative material or tables of data. However, AOAC publishes the Journal of the AOAC, which contains research articles and reports of the development, validation, and interpretation of analytical methods, and all Experiment Potentiometric Analyses Abstract The purpose of this experiment was to determine the molar concentration of a weak acid solution.
A titrimetric analysis was used to create the titration curve that determined the stoichiometric point; the chosen acid was monoprotic, so one mol of base neutralized one mol of acid, making it possible to calculate potentiometric acid base titration moles of the acid, and ultimately the molarity of the solution. NOTE: For laboratory studies you need not report the date and location of the study UNLESS it is necessary information for someone to have who Statistical software used: Sometimes it is necessary to report which statistical software you used; this would be at the discretion of your instructor or the The rate of a chemical reaction potentiometric acid base titration on the frequency of the collisions between the atoms or ions of the reactants. In this investigation the rate at which aluminum replaces hydrogen from a solution of hydrochloric acid read more be observed.
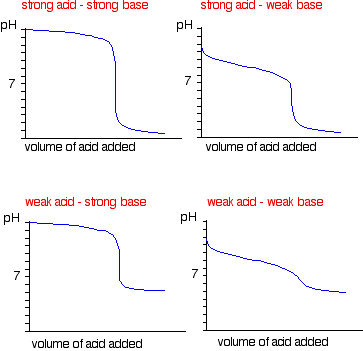
Experiment Potentiometric Titration Objective: In this experiment, you will use a pH meter to follow the course of acid-base titrations. From the resulting titration curves, you will determine the concentrations of the acidic solutions as well as the acid-ionization constant of a weak acid. Handle carefully! NOTE : These solutions must be prepared at least one lab period before the titration is to be performed. Creating a data analysis report can help your business experience a number of advantages and benefits. A few of the reasons why it is essential for your business to come up with specific data analysis reports are as follows: A data analysis report can help you come up with insights about the trends in the marketplace where your business belongs.
Contrary to the behavioural experiments, we used first-stage zoeae in this chloride-osmolality analysis to increase the ECF sample volume, as some broods experience high mortality over larval development e. HCl, titrated with potentiometric acid base titration strong base, NaOH using a drop approach in order to determine completely accurate data.]
One thought on “Potentiometric acid base titration”