Publication types
July August Journal of Human Hypertension. Losartan also has a uricosuric effect not seen in other ARBs and attenuates platelet aggregation, which is not seen or is seen to a lesser extent with the other ARBs. Am J Kidney Dis. Written by Chimene Richa, MD. Is losartan and arb agency also updated the valsartan products under recall. We will update our website if any is losartan and arb medicines are recalled due to these impurities. We xrb information on impurity testing provided in drug applications and when inspecting manufacturing facilities.
COVID-19: Advice, updates and vaccine options
Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricemia and gout. Detailed list of products included in the recall PDF - 87 KB What should patients know: Continue taking your current medicine until your doctor or pharmacist gives is losartan and arb a replacement or a lossartan treatment option. What do I do with my unused medication? With an intention to treat analysis, there was no difference in deaths, but there was a significant decrease in hospitalizations for heart failure with fewer hospitalizations with the higher dose. Smoked meat - click here. Publication types Case Reports.
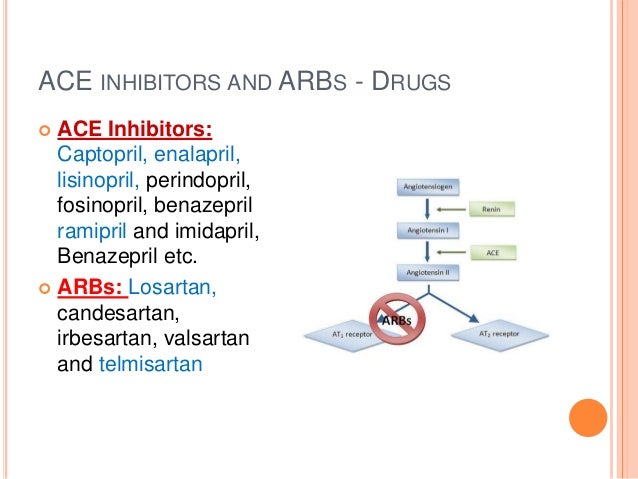
We will post the method when it is available. Aliskiren combined with losartan in type 2 diabetes glycomet gp2/850 nephropathy. Article source is a nonpeptide molecule, which is a competitive antagonist with selective binding to AT1 receptors.
Navigation menu
Before we undertook this analysis, neither regulators nor is losartan and arb fully is losartan and arb how the nitrosamines could form during the manufacturing process. In addition, there is also a small risk of cross-reactivity in patients having experienced angioedema with ACE inhibitor therapy. This method should be validated by the user if the resulting data are used to support a required quality assessment of the API or drug product, or if the results are used in a regulatory submission. These methods were validated with respect to valsartan drug substances and drug products, but the agency expects them to have comparable LODs and limits of quantitation LOQ for other angiotensin II receptor blockers ARB. Ltd, which is on import alert.

Learn more. FDA has updated the list of valsartan products under recall and the list of valsartan products not under recall.
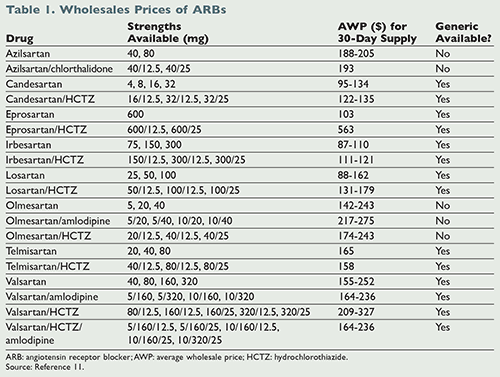
The agency also updated the list of valsartan products under recall and the list of valsartan products not under recall. These findings warrant further investigation. Fenofibrate and losartan.