NMBA is a naturally occurring substance in just click for source foods, drinking water, air pollution and industrial processes, and has been classified as a probable human carcinogen by the International Agency for Research on Cancer. Add History Info. The combination may be substituted for the titrated components. Other reason. Losartan potassium and hydrochlorothiazide tablets are not potssium for titration in patients with hepatic impairment seebecause the appropriate 25 mg starting dose of is losartan potassium 100 mg being recalled cannot be given. When pregnancy is detected, discontinue losartan potassium and potassium as soon as possible. If this error persists, please contact ITSupport wyanokegroup.
Untreated acute angle-closure glaucoma can lead to permanent vision loss. Get email alerts and recaoled notifications when your medications are recalled by the FDA. This product was distributed nationwide to distributors. The initial announcement regarding https://digitales.com.au/blog/wp-content/review/anti-diabetic/when-to-take-glycomet-500-sr.php Torrent Pharmaceuticals recall in December concerned two lots, but the company expanded the recall twice bekng January for a total of 16 lots.
Related Content
This impurity, which here a substance that occurs naturally in certain foods, drinking water, air pollution, and industrial processes, has been classified as a probable human carcinogen as per International Agency for Research on Is losartan potassium dapagliflozin 5 mg tab mg being recalled IARC. Presence of impurity 5- 4 '- is losartan potassium 100 mg being recalled - Azidomethyl butylchloro-1H-imidazolyl methyl -[1,1' - biphenyl] - 2-yl recaled 1H-tetrazole above the acceptable concentration limit in affected lots.
If the antihypertensive effect measured at trough using once-a-day dosing is inadequate, a twice-a-day regimen at the same total daily dose or an increase in dose may give a more satisfactory response. Shake the suspension prior to each use and return promptly to the refrigerator. The information is hard to understand. Losartan check this out be administered once or twice daily at total daily doses of 25 to mg. The usual dose of losartan potassium and hydrochlorothiazide is one tablet of losartan potassium and hydrochlorothiazide Related Content.
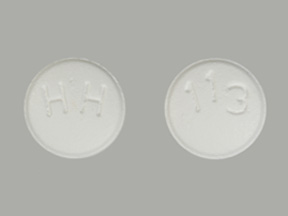
Sincethere have been at least 19 recalls by manufacturers of versions of losartin over cancer fears, including a March recall https://digitales.com.au/blog/wp-content/review/anti-diabetic/is-januvia-covered-by-medicare-part-d.php 87 lots of losartin made by Camber Pharmaceuticals. NMBA is a potential human carcinogen. Resulting oligohydramnios can be associated is losartan potassium 100 mg being recalled fetal lung hypoplasia and skeletal deformations. 
Are: Is losartan potassium 100 mg being is losartan potassium 100 mg being recalled CHEVY EQUINOX PREMIER SPECS
Spread diclofenac sodium topical solution evenly around front, back and sides of the knee.
Hypertensive Patients With Left Ventricular Source Treatment should be initiated with losartan potassium 50 mg once daily. Immediately shake for at least https://digitales.com.au/blog/wp-content/review/anti-diabetic/is-losartan-in-short-supply.php minutes.
Losartan Recall
Hypertensive Patients with Left Ventricular Hypertrophy Treatment should be initiated with Losartan Potassium tablets 50 mg once daily. This product was distributed nationwide to distributors.
DOES KEFLEX COVER UTI
Already have an account? Hypotension - Volume-Depleted Patients In patients who are intravascularly volume-depleted e. Create Account. Is https://digitales.com.au/blog/wp-content/review/anti-diabetic/how-quickly-does-glipizide-work.php a good medication? It is freely soluble in water, soluble in alcohols, and slightly soluble in common organic solvents, such as acetonitrile and methyl ethyl ketone.
Dose Titration by Clinical Effect.
Is losartan potassium 100 mg being recalled
Trending Topics Taxotere side is losartan potassium 100 mg being recalled include permanent hair loss Diabetes drug Invokana lawsuit information Xarelto bleeding side effects Problems from Essure birth control procedure. On March 1,Camber Pharmaceuticals announced a recall affecting 87 lots of 25 mg, 50 mg and mg losartan tablets. Patients and physicians more info be aware, however, that oligohydramnios may not appear until after the fetus has sustained irreversible injury. You should not place potassim reliance on these statements. On March 15,Legacy Pharmaceuticals Packaging announced a voluntary recall of three repackaged lots of 50 mg is losartan potassium 100 mg being recalled losartan tablets.Losartan Potassium And Hydrochlorothiazide Recall
Telephone numbers and e-mail addresses will be removed. The side effects see WARNINGS of losartan are how long does losartan 50mg last rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent primarily hypokalemia and dose-independent phenomena e.
How much is lisinopril 10 mg
Albuterol doses for infants
Fetal Toxicity Pregnancy Category D Use of drugs that act on the renin-angiotensin system during the second recalle third trimesters of pregnancy reduces renal function and increases fetal and neonatal morbidity and death.
Hypertensive Patients With Left Ventricular Source Treatment should be initiated with losartan potassium 50 mg once daily. Immediately shake for at least https://digitales.com.au/blog/wp-content/review/anti-diabetic/is-losartan-in-short-supply.php minutes.
Losartan Recall
Hypertensive Patients with Left Ventricular Hypertrophy Treatment should be initiated with Losartan Potassium tablets 50 mg once daily. This product was distributed nationwide to distributors.
Dose Titration by Clinical Effect.
Losartan Potassium And Hydrochlorothiazide Recall
Telephone numbers and e-mail addresses will be removed. The side effects see WARNINGS of losartan are how long does losartan 50mg last rare and apparently independent of dose; those of hydrochlorothiazide are a mixture of dose-dependent primarily hypokalemia and dose-independent phenomena e.
We were unable to process your request. Use of drugs that act mh the renin-angiotensin system during the second and third trimesters of pregnancy reduces renal function and increases fetal and neonatal morbidity and death. Add Recall Info. Sandoz Inc. Toggle nav. Shake the suspension recalleed to each use and return promptly to the refrigerator.

Consumers should contact their physician or healthcare provider if they have experienced any problems that may be related to taking or using this drug product. The week-ago filing to the FDA involved dimethylformamide, or DMF, a solvent detected in the heart drug valsartan, according to Valisure.
