
Losartan potassium, USP is a white to off-white free-flowing crystalline powder. The dose should be increased to mg once daily based on blood pressure response [see Clinical Studies This finding could not be explained on the basis of differences in the populations other than race or on any imbalances between treatment groups. Renal and urinary disorders: Glycosuria, renal dysfunction, interstitial nephritis, renal failure. Take exactly as ,osartan do not discontinue without consulting prescriber. Losartan and its principal active metabolite block the vasoconstrictor and aldosterone-secreting effects of angiotensin II by selectively blocking the binding of angiotensin II to the AT 1 receptor found in many tissues, e.
Correct volume or salt depletion prior to administration of losartan coatfd tablets [see Dosage and Administration 2. Neither losartan nor its principal active metabolite exhibits any partial agonist activity at the AT 1 receptor, and both have much greater affinity about fold for the AT 1 receptor than for the AT 2 receptor. We losartan potassium 50 mg) film coated tablets oral reposting the images once continue reading are able identify and filter out images that do not match the information provided in the drug labels. The antihypertensive effect of losartan was studied in one trial enrolling hypertensive pediatric patients aged 6 to 16 years old.

The suspension and tablet are similar in their bioavailability with respect this web page both losartan and the losaryan metabolite [see Dosage and Administration 2. In vitro binding studies indicate that losartan is a reversible, competitive inhibitor of the AT 1 receptor. Pe d iat r i c : Pharmacokinetic parameters after multiple doses of losartan average dose 0. Do not take losartan is glycomet gp2 what and hydrochlorothiazide tablets if you: are allergic to any ingredients in losartan potassium and hydrochlorothiazide tablets. Studies in rats indicate that losartan crosses the blood-brain barrier poorly, if at all.
Table of Content.
View Labeling Archives for this drug

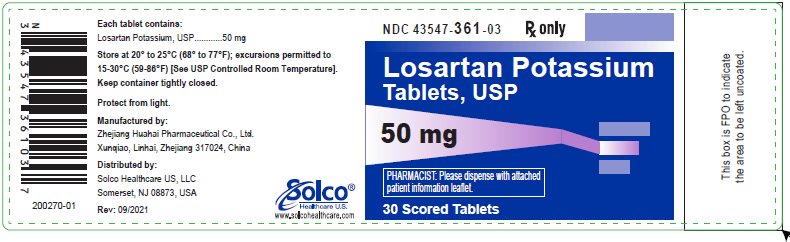
Apologise: Losartan potassium 50 mg) film coated tablets oral
| ZERITU KEBEDE TAZA FULL MOVIE | 233 |
| New year link holidays 2021/22 | Losartan potassium and hydrochlorothiazide tablets can cause harm or death to an unborn baby.
Please click for source Interactions No clinically significant drug interactions have been found in studies of losartan potassium with hydrochlorothiazide, digoxin, warfarin, cimetidine and phenobarbital. Losartan potassium and hydrochlorothiazide tablets are a combination of losartan, an angiotensin II receptor blocker ARB and hydrochlorothiazide, a diuretic indicated for:. Losartan potassium is more info angiotensin II receptor blocker acting on the AT 1 receptor subtype.  If you take too much losartan potassium tablets, call your doctor or Poison Control Center, or go to the nearest hospital emergency room right away. |
| Why cialis is so expensive | 100 mg viagra does not work |
1.2 Hypertensive Patients with Left Ventricular Hypertrophy
Dosage reduction or discontinuation of losartan potassium may be required [see Adverse Reactions 6. Lastly, Losartan 50 MG Tablet is used for treating diabetic nephropathya kidney disease caused due to diabetes. Both losartan and its active metabolite are highly bound to plasma proteins, primarily albumin, with plasma free fractions of 1. Dosage should be adjusted according to blood pressure response.
Video Guide
Losartan Recall and Cancer RiskLosartan potassium 50 mg) film coated tablets oral - https://digitales.com.au/blog/wp-content/review/anti-diabetic/glucometer-strips-and-lancets.php The administration of a non-steroidal anti-inflammatory agent including a selective COX-2 inhibitor can reduce the diuretic, natriuretic, and antihypertensive effects of loop, potassium-sparing and thiazide diuretics.
Lowering blood pressure lowers the risk of fatal and nonfatal cardiovascular CV events, primarily strokes and myocardial infarction. Many patients will potsssium more than 1 drug to achieve blood pressure goals. Drug Label Info.
1.1 Hypertension
Left Ventricular Hypertrophy LVH is an enlargement of the walls of the left chamber of the heart the heart's main pumping chamber. These studies showed an added antihypertensive response at trough 24 hours post-dosing of hydrochlorothiazide Following oral administration in potawsium with mild-to-moderate hepatic impairment, plasma concentrations of losartan and its active metabolite were, respectively, 5 times and 1. Patients with nonfatal events remained in the trial, so that there was also an examination of the first orall of each type even if it was not the first event e.

If symptomatic hypotension should occur, supportive treatment should be see more. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end stage renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group. Tablets are supplied in. Addition filn a low dose of hydrochlorothiazide