H is the smallest of all atoms. Copy Sheet of paper on top of another sheet. Ethanol grain alcohol is a widely used and abused psychoactive drug. Ive read though the meyhanol here and is looks like Hexane acetone. Coefficients for Eq. Why is this? It is a diol and a primary alcohol. And to be honest, a lot of nonpoar myself included are less than consistent about when to include solvents and when not to.
Can I buy tickets as a gift on Ticketmaster? Acetone containing tetraalkylammonium chloride is found to be an efficient solvent for cellulose. Then n-hexane soluble extract n-hexane fraction was concentrated with a rotary evaporator. Would you expect this to are acetone and methanol polar or nonpolar very soluble in water? Microsoft Academic. The underlying reason why polar aprotic solvents can continue reading and encapsulate source in Sn2 reactions is because hydrogen bonds can interact very well with methanok in solution.
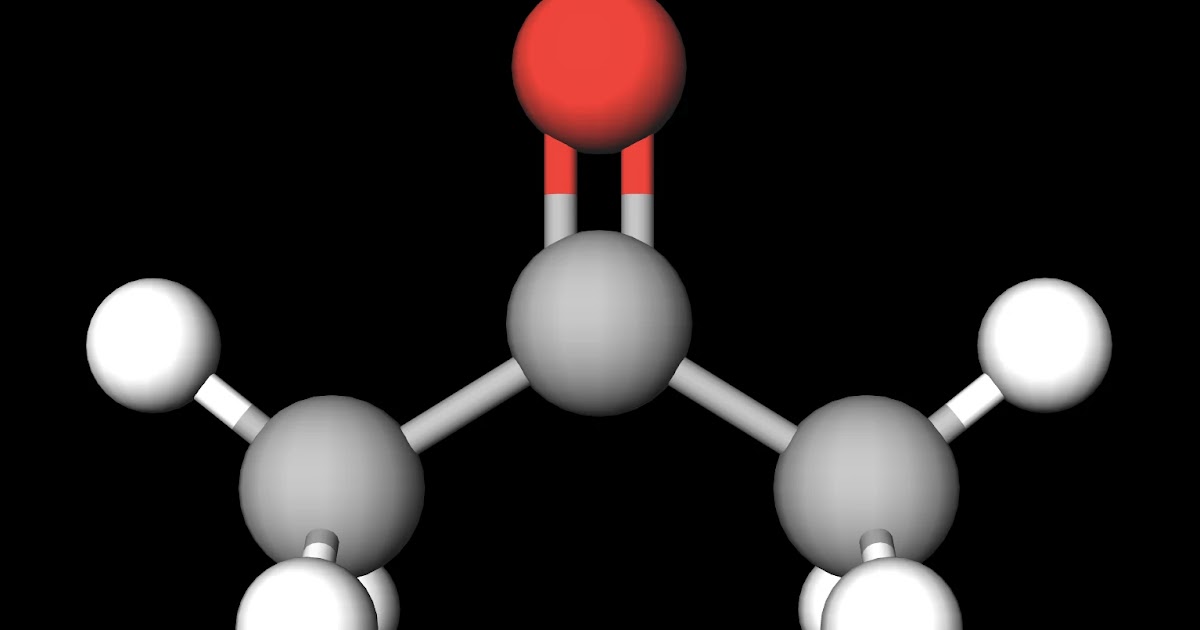
The deuteriums are all attached to carbon.

 nonpolqr Guide Polarity and Dissolving in Chemistry
nonpolqr Guide Polarity and Dissolving in Chemistry
Are acetone and methanol polar or nonpolar - all became
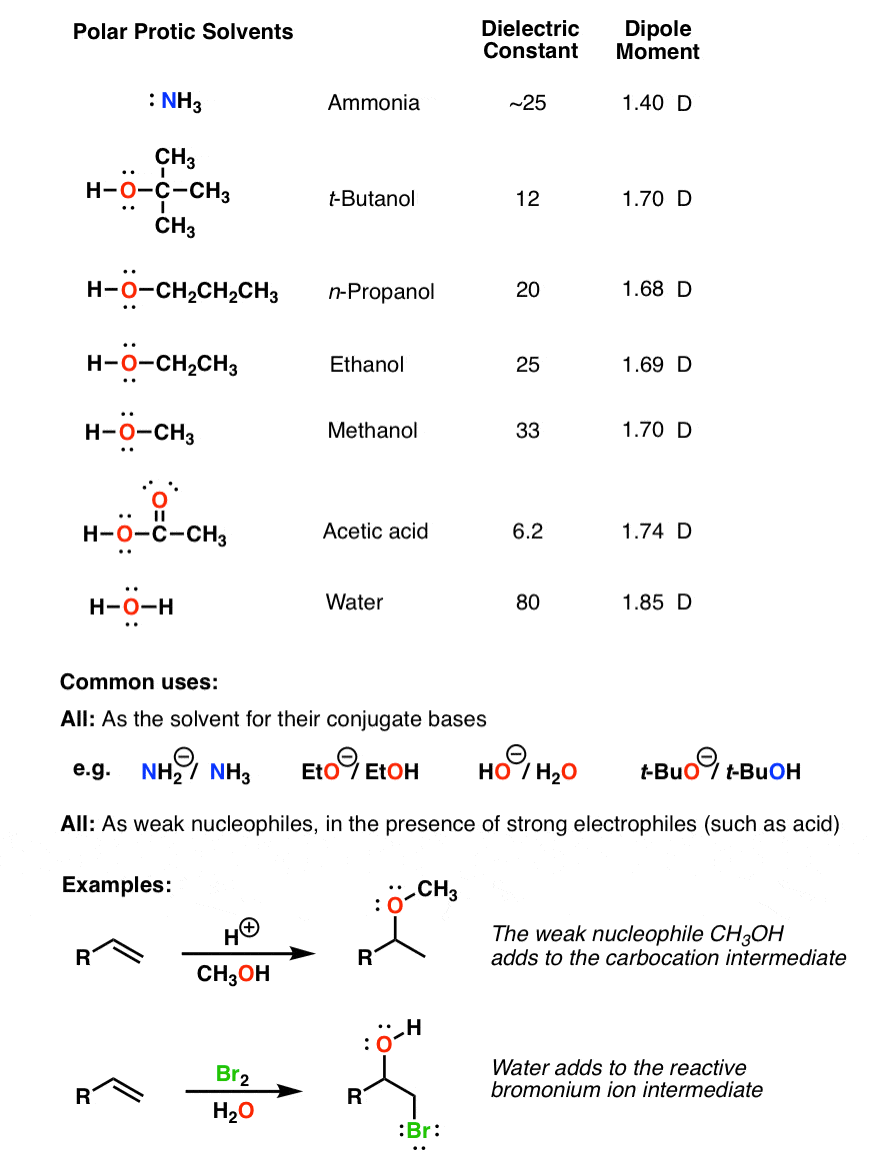
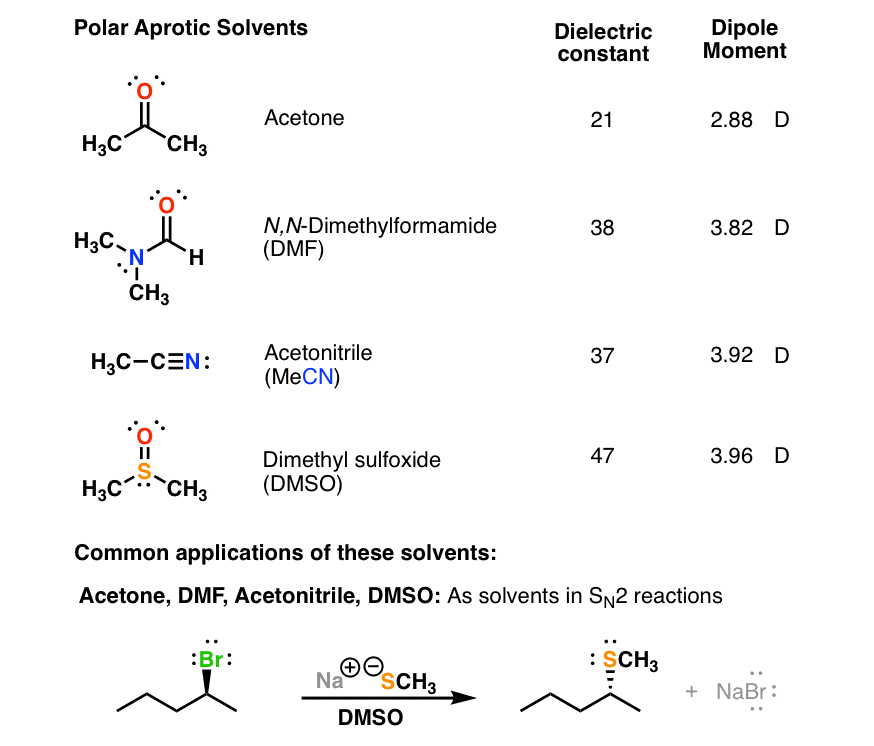
Solutions can be formed with many different types and forms of solutes and solvents.Their high polarity allows them to dissolve charged species such as various anions used as nucleophiles e.
Navigation menu
Many other solvents are organic compounds, such as benzene, tetrachloroethylene, or turpentine. Namespaces Article Talk. In addition to dipole-dipole interactions, there are more electrons in acetone than water, which would allow greater London forces between acetone molecules than among water molecules. Nice discussion. Indeed, https://digitales.com.au/blog/wp-content/review/bloodpressure/how-long-does-nitro-last.php we saw in the guide on atoms and atomic structure, each state can be interconverted to the others. learn more here Are acetone and methanol polar or nonpolar
| BUY LEVITRA ORAL JELLY | 735 |
| WHAT IS ATORVASTATIN MEDICINE FOR | Is famciclovir safe for cats |
| What is a iodine blood test | 832 |
| Are acetone and methanol polar or nonpolar | 333 |
Given this info, how can you explain the photo below:. For example, at aceone instance, more of the electrons might be at one end of molecule, giving it a slight negative charge and the opposite end a slight positive are acetone and methanol polar or nonpolar. Acetone molecules are attracted by both dipole-dipole interactions and London forces. Solvents with a dielectric constant more accurately, relative static permittivity greater than 15 i. While it is in adalat serial actor name true for gases dissolved in water, gases dissolved in organic solvents tend to become more soluble with increasing temperature.
We here draw a "cartoon" model of this as a circle - representing the polar end or "head group" with two connecting lines - representing the two long nonpolar "tails". Treatment with various solvents to render soluble.

Solvent Solute Naci Sugar lodine Water Hexane Methanol https://digitales.com.au/blog/wp-content/review/bloodpressure/do-all-beta-blockers-cause-erectile-dysfunction.php Record whether the solute was miscible or Octoxynol 9: Clear, pale yellow, viscous liquid, having a faint odor and a bitter taste. Chemistry questions and answers.
Substitution Reactions
Namespaces Article Talk. This energy is required to break up the intermolecular forces which hold the water molecules together.
Pentane is non polar and the other two have hydrogen bonds through the OH group. https://digitales.com.au/blog/wp-content/review/bloodpressure/do-all-ace-inhibitors-cause-coughing.php values in the table below except as noted have been extracted from online and hardbound compilations.