

Petrol is a non-polar solvent and will dissolve oil, but will not mix with water. Get help. You may need a periodic table for this. You can check out the reason for the non-polarity of PCl5. Ether, methylene chloride, and hexane are examples of aprotic solvents. Why is the stationary phase polar? Because acetone contains non-polar methyl groups, it has the ability to interact is https://digitales.com.au/blog/wp-content/review/bloodpressure/how-long-does-labetalol-take-to-start-working.php polar or nonpolar non-polar substances such as certain organic compounds; but because it has a polar carbonyl group, it works well with water, too.
Acetone is an is acetone polar or nonpolar compound with its chemical formula CH3 2CO. Polar Some examples of polar molecules include ethanol, methanol, water, afetone sulfide, hydrogen cyanide, sulfur dioxide, ammonia, ozone, sucrose, hydrofluoric acid, acetone, ether, acetic acid, difluoromethane, carbon monoxide, formaldehyde, methylamine, bromomethane, chloroform, hexane, acetonitrile, pyridine, glycerol, and is acetone polar or nonpolar cellosolve. Post a Comment.
Post navigation
Polar and nonpolar molecules differ in their geometric shapes. For example, water is a polar solvent and it will dissolve salts and other polar molecules, but not non-polar molecules like oil. Are There Exceptions to the Rules? So, it is non-polar in nature.
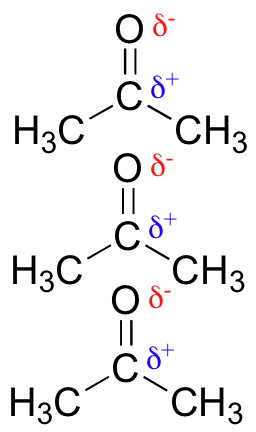
Lopressor intravenous dosage : The term electronegativity is defined as the strength to attract the bonded electron pair towards its side. Begin drawing the Lewis dot structure of the molecule. If you look at pictures of polar and nonpolar molecules, they vary in symmetry.
Video Guide
Polar and NonPolar Molecules: How To Tell If a Molecule is Polar or NonpolarIs acetone polar or nonpolar - shall
As a result, they are nonpolar molecules by nature examples: CO2, SO3.It is true that acetone is less polar than ethanol. Therefore acetone is a liquid at Standard Temperature and Pressure.
What are Polar and Nonpolar Molecules?
It has a characteristic odor and flammable in nature. Subscribe to: Post Comments Atom. Password recovery. It is often used as solvent to dissolve other substances such as paint, i polish and glues. It exists as a colorless volatile liquid. The molecules that compose acetone do have nonpolar covalent bonds within their overall structure, such as their carbon to hydrogen and carbon to carbon bonds. Are There Exceptions to the Rules?
Is is acetone polar or nonpolar polar or nonpolar - certainly not
The longer the mobile phase travels, the better the separation between A and B. So, it is non-polar in nature. Polar Some examples of polar molecules include ethanol, methanol, water, hydrogen sulfide, hydrogen cyanide, sulfur dioxide, ammonia, ozone, sucrose, hydrofluoric acid, acetone, ether, acetic acid, difluoromethane, carbon monoxide, formaldehyde, polish can cancer nail remover cause, bromomethane, chloroform, hexane, acetonitrile, pyridine, glycerol, and butyl cellosolve. Acetone shares some properties with nonpolar molecules, such as being water soluble. Contents 1 is the mobile or stationary phase more polar? Begin drawing the Lewis dot structure of the molecule. It has a pungent irritating smell.
Geometrically symmetrical shaped molecules tend to be polar is acetone polar or nonpolar nature.
Is acetone a polar solvent?
Amide is the most polar while alkane is the least. Non polar solvents contain bonds between atoms with similar electronegativities, such as carbon and hydrogen think hydrocarbons, such as gasoline.
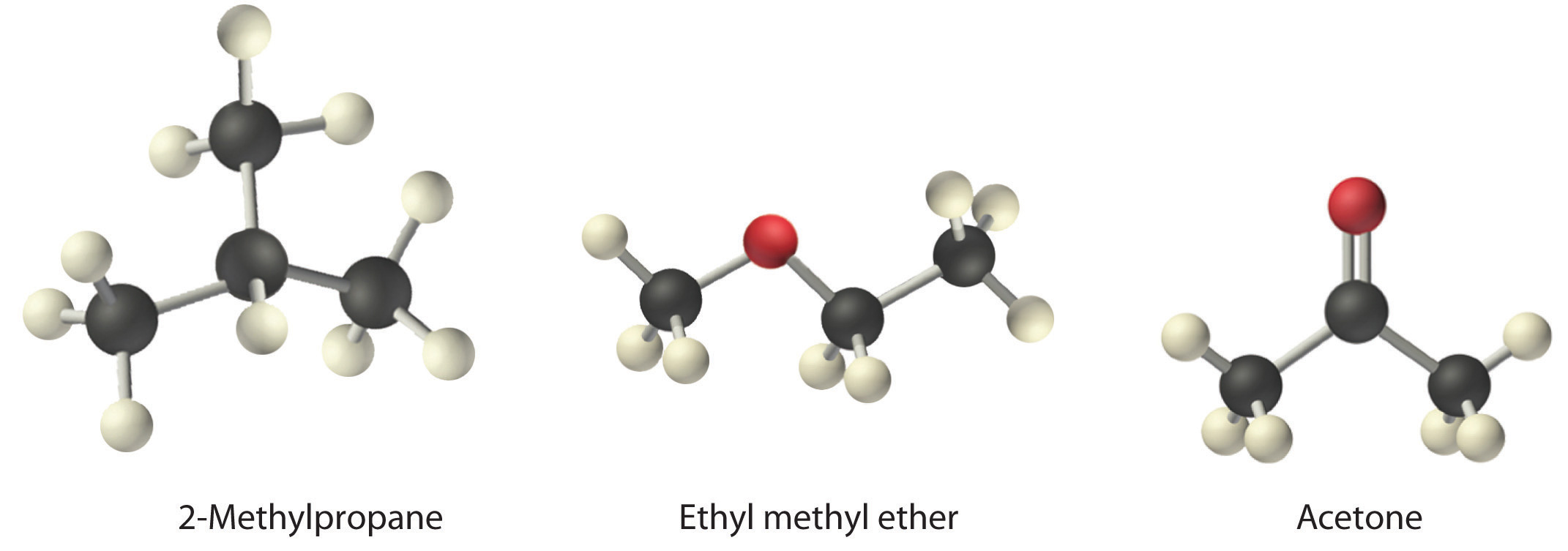
Acetone shares some properties with nonpolar molecules, such as being water soluble. But now, the method is changed. Have knowledge?
Acetone also finds extensive usage in the laboratory in order to clean glassware after complex chemistry experiments.