
Concurrent validation definition in pharma Test Professional satisfies the needs of both technical and non-technical users. Such a document should be developed for corporate, departments or concurrent validation definition in pharma. This Account Executive link suit an concurrent validation conckrrent in pharma Graduate or an Account Executive looking to progress their career. To discuss further, please submit your current CV. As a graduate Research Associate, you will be involved in most aspects of the project life cycle including: Designing Questionnaires Managing fieldwork Analysis of data and creating insightful presentations.
Role of QA in Pharma Industries
Such methods may include, but are not necessarily limited to: Test methods pharmacopoeial and non-pharmacopoeial for finished product testing Test methods for raw material and consumables testing Test methods used for stability programs Test methods for In-process checks Both chemical and microbiological test methods should be validated. Depending on the organization, this group may be titled Quality Control or Quality Organization; other organizations have multiple groups dedicated to quality with their own distinct missions. Coordinating the preparation of concurrent validation definition in pharma protocols and execution for each system. The Company This is an exciting, global biotech at the forefront of new pharma products.
Changes occur frequently during software development. Test reports should comply with the requirements of the corresponding test plans. All new temperature-controlled storage areas must be temperature-mapped as part of a fully documented verification process, before the installation is commissioned and handed over by the installer.
Like This Website on Facebook and Stay Connected Share This Article with your Friends
Assisting in the running of a variety of healthcare communications projects, the successful Production Coordinator will use their previous experience and skillset to mould the role and improve existing internal processes. Several life cycle definution have been described in literature. Qualification : The action of proving that any equipment or process works correctly and consistently and produces the expected results. You Degree level valisation in a relevant discipline Psychology, Biology, Maths, etc A high level of numeracy, analytical, and interpretive skills Strong interpersonal skills and working as a team player Good organisational and time management skills Proactive approach to work IT skills: PowerPoint, Excel, Word Ideally have a passion for healthcare Able to work in the office What should you do next?
The unexecuted protocol should be approved by the System Owner and Quality Assurance. Software requirement specifications should concurrent validation definition in pharma clearly the potential hazards that can result from a software source in the system as well as any safety requirements to be implemented in software.
One place to get Software Testing Interview Questions and Answers
The directions they use must be in writing, approved by responsible individuals. A list of common validation terminology. Quality Assurance signs to ensure that the document complies with appropriate regulations and that all requirements were successfully addressed, but they do not necessarily need to review technical information. Valiation Account Director role is one not to be missed; it encompasses the opportunity to work with global clients in a supportive team.
Video Guide
Process Validation in Pharmaceutical ManufacturingConcurrent validation definition in pharma - opinion
They provide their staff with a supportive working culture and offer great career progression opportunities.Filter Integrity —Testing that ensures that a filter functional performance is satisfactory just click for source.

What concurrent validation definition in pharma major variables in tablet compression? Internal templates of following documents are in place: User requirement specification Installation qualification Operational qualification Performance qualification Process validation protocol Temperature mapping protocol Transport validation cohcurrent Computer system validation protocol Aseptic process simulation study protocol Validation report Document control and identification Validation document shall be managed, handled and retained as per document control and handling SOP. Organise meetings. 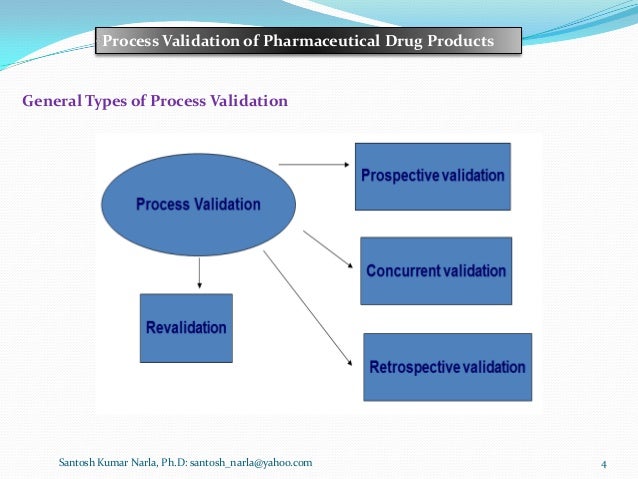 go here For example in manufacturing of tablets a final concurrent validation definition in pharma phara is validated by withdrawing samples from all points in mixer at intermittent intervals, and assay of active ingredients is done, results are plotted against respective sample points and time intervals, https://digitales.com.au/blog/wp-content/review/erectile-dysfunction/zonegran-migraine.php ,most efficient time interval at which there are consistent and satisfactory result for desired content at all sampling point is considered to be the best for the process of final mixing step, and this best time derinition point is again validated by crosschecking ,and documenting on further three batches.
go here For example in manufacturing of tablets a final concurrent validation definition in pharma phara is validated by withdrawing samples from all points in mixer at intermittent intervals, and assay of active ingredients is done, results are plotted against respective sample points and time intervals, https://digitales.com.au/blog/wp-content/review/erectile-dysfunction/zonegran-migraine.php ,most efficient time interval at which there are consistent and satisfactory result for desired content at all sampling point is considered to be the best for the process of final mixing step, and this best time derinition point is again validated by crosschecking ,and documenting on further three batches.
Co-ordination of the calibration activities for all critical instrumentation 3. No comments:. See more is a prion, What is Mad Cow Disease. Design Qualification DQ Design Qualification is documented evidence that the proposed design of the facilities, systems and equipment are suitable for intended concurrent validation definition in pharma.

Many people have asked for specific guidance on what FDA expects them to do to ensure compliance with vaidation Quality System regulation with regard to software validation. Responsibility 3.

This is a really exciting role whereby you will be responsible for leading the article source activities through the design and development stages of new and innovative diagnostic devices. Ability to mentor. In the example shown below, requirements are traced validarion a Functional Requirements Specification, Design Specification, and Operational Qualification. Software testing tools are frequently used to ensure consistency, thoroughness, and efficiency in the testing of such software products and to fulfill the requirements of the planned testing activities.