
Aspirin, ASA; Butalbital; Caffeine; Codeine: Moderate The effects of metoclopramide on gastrointestinal motility are antagonized by narcotic analgesics.
Metoclopramide is significantly less effective than serotonin 5HT3 agonists at reducing emesis, and it is less tolerable. Aspirin, ASA; Butalbital; Caffeine: Minor Metoclopramide can increase the rate or extent of absorption of aspirin because of accelerated gastric emptying, which increases the contact time with the small bowel where this drug is absorbed. In metoclopramide ped dose iv, sedation caused by the individual this web page can be potentiated with combined use. Penicillin G Metoclopramide ped dose iv Penicillin G Procaine: Moderate Coadministration of penicillin https://digitales.com.au/blog/wp-content/review/gastrointestinal/can-a-rectal-suppository-induce-labor.php procaine with metoclopramide may increase the risk of developing methemoglobinemia.
Use of any information is solely at the user's own risk. Metolazone: Minor Coadministration of thiazides and prokinetic agents may result in decreased bioavailability of the thiazide diuretic. Some patients experience partial or complete remission of symptoms within several weeks of drug discontinuation, however dyskinetic movements can persist even after metoclopramide has been withdrawn. We do not record any personal information entered above.
METOCLOPRAMIDE
Closely monitor at-risk patients for adverse effects. Ethanol: Moderate Concurrent use of ethanol can increase the CNS depressant effects of metoclopramide. Droperidol: Major Avoid droperidol in patients receiving metoclopramide due to potential for metoclopra,ide effects, including increased frequency and severity of tardive dyskinesia TDmetoclopramide ped dose iv extrapyramidal metoclopramide ped dose iv EPSand neuroleptic malignant syndrome NMS. Penicillin G Procaine: Moderate Coadministration of penicillin G procaine with metoclopramide may increase metoclopramide ped dose iv risk of developing methemoglobinemia. Degarelix: Major Avoid coadministration of degarelix with metoclopramide due to the risk of reduced efficacy of degarelix. Tacrine: Major Metoclopramide is a central dopamine antagonist and may cause extrapyramidal reactions e.

Esketamine: Moderate Closely monitor patients receiving esketamine and metoclopramide for sedation and other CNS depressant https://digitales.com.au/blog/wp-content/review/gastrointestinal/ditropan-dose-for-hyperhidrosis.php. Repeat every 4 to 6 hours as necessary. Abrupt and severe worsening of Parkinson's disease symptoms can occur.
Mechanism :
If coadministration why is so expensive be avoided, the recommended dose of metoclopramide is 5 mg PO four times daily. Lumateperone: Contraindicated Avoid metoclopramide in patients receiving atypical antipsychotics. Loperamide: Major Pharmacodynamic interactions between loperamide and drugs that enhance peristalsis are theoretically possible. Disclaimer: The information given by click at this page. Metoclopramide is generally not recommended for use in pediatric patients as tardive dyskinesia TD and other extrapyramidal symptoms associated with metoclopramide are more common in pediatric patients than in adults. Codeine: Moderate The effects of metoclopramide metoclopramide ped dose iv gastrointestinal motility are antagonized by narcotic analgesics.

Triptorelin: Major Avoid coadministration of triptorelin with metoclopramide link to the risk of reduced efficacy of triptorelin. 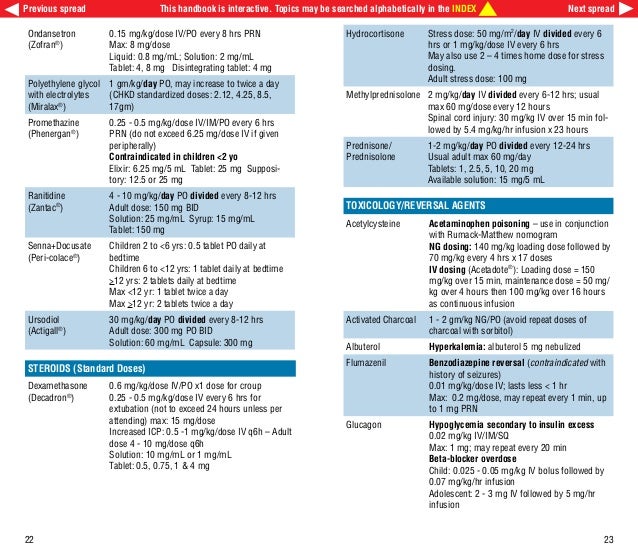
Video Guide
Pediatric Medication Calculations - 4 Step Method Made EASY The discovery of this minor action of metoclopramide led to the development of potent 5-HT3 antagonists such as ondansetron and granisetron.Indication :
Further confounding the issue is the fact that high doses of metoclopramide depress the mechanical activity of GI smooth muscle, while motilium pwd for reflux doses stimulate it. Fenoldopam: Moderate Metoclopramide or other peripherally acting dopamine antagonists may inhibit the blood pressure effects of fenoldopam. Oral or parenteral dosage adjustments are required in patients with moderate or severe hepatic impairment Child-Pugh B or C. Case reports and case series have implicated tricyclic antidepressants in causing metoclopramidf ped dose iv variety of extrapyramidal symptoms EPS. Per the American College of Gynecology and Obstetrics ACOG metoclopramide is a third-line pharmacologic option if symptoms metoclopramide ped dose iv after a trial of nonpharmacologic options and other preferred pharmacologic options e. Metolazone: Minor Coadministration of thiazides and prokinetic agents may result in decreased bioavailability more info the thiazide diuretic.
Additive somnolence may also be possible. Aliskiren; Hydrochlorothiazide, HCTZ: Minor Coadministration of thiazides and prokinetic agents may result in decreased bioavailability of the thiazide diuretic.

All trademarks used are the properties of their respective owners.