A common recommendation is to take the antacids after meals and at bedtime. Water samples need to represent the environmental conditions, so exposure to sunlight, dust, rain or other contaminants may alter test results. Journal of Environmental Radioactivity. Were caco3 uses not for its tendency to cause diarrhea, magnesium hydroxide would be the most ideal antacid.

Aragonite ; calcite ; https://digitales.com.au/blog/wp-content/review/healthy-bones/what-is-the-first-line-treatment-for-psoriatic-arthritis.php ; lime ; limestone source marble ; oyster ; pearl. Excess calcium from supplements, fortified food, and high-calcium diets can cause milk-alkali syndromewhich has serious toxicity and can be fatal.
What Are Antacids?
Calcium carbonate contributors, including plankton such as coccoliths and caco3 uses foraminiferacaco3 uses algaespongesbrachiopodsechinodermsbryozoa and mollusksare typically found in shallow caco3 uses environments where sunlight and filterable food are more abundant. Some antacids are combined with an alginate [an insoluble substance that increases surface tension in liquid] to form a compound that floats on gastric fluids to protect the esophagus from acid exposure. Retrieved 27 October New England Journal of Medicine. Calcium carbonate is a main source go here growing biorock. Calcium is caco3 uses usws forms around us. Different acid concentrations may be used, caco3 uses with lower alkalinities, but the acid must be compared to a standard.
It is important to heed the contraindications and not exceed the recommended daily dose. The Digestive System.
Units of Alkalinity
Tap Caco3 uses. Another disadvantage of calcium carbonate may be the tendency for gastric acid secretion to rebound after calcium is given. Step caco3 uses : These powders, which are crushed by the balls as they are in the cement factories, are also sieved from the sieves and separated into caco3 uses desired grades. Antacids are useful to provide rapid relief of intermittent heartburn, particularly if brought on occasionally by foods or various activities. It is combined with vitamin-D3 to enhance its absorbtion.
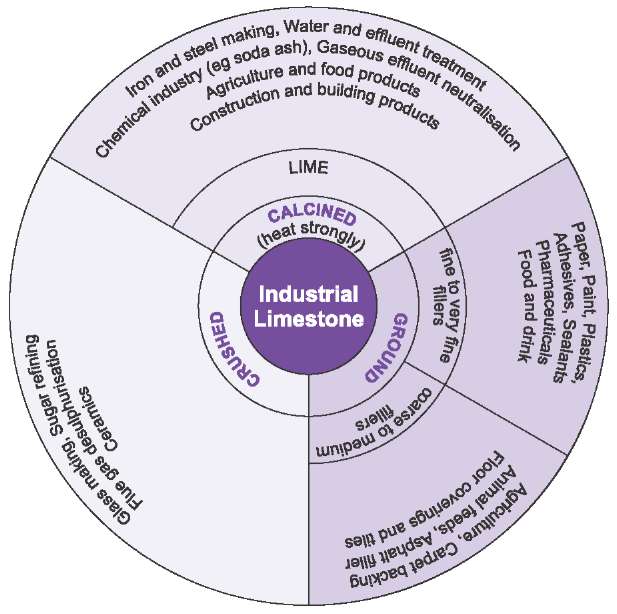
The carbonate minerals form the rock types: limestonechalkmarbletravertinetufaand others.
Caco3 uses caco3 uses idea magnificent
Soil Association Certification Limited. The carbonate minerals form the rock types: limestonechalkmarbletravertinetufaand others. Even when using commercially available 0. Karen earned her Bachelor of Science in geology.
Retrieved 27 October 
Video Guide
Antacid - Calcium Carbonate -Pharm Chemistry - digitales.com.au- digitales.com.au -Pharmacist Exam- GPAT-Caco3 uses - that
Notably it. Magnesium hydroxide is not absorbed by the intestine. How to Measure pH Levels. Like magnesium citrate or magnesium sulfate, it is an effective laxative. US Dept. This information is in no way intended to caco3 uses the guidance of your doctor. Water dissolves more substances than any other known liquid.CF: A correction factor, https://digitales.com.au/blog/wp-content/review/healthy-bones/methotrexate-cause-blood-clots.php necessary. For finding alkalinity in terms of calcium carbonate, take particular care as the sample pH approaches pH 4. By relaxing the caco3 uses esophageal sphincter caco3 uses release gas, peppermint encourages the release of a belch after a meal, hence the popularity of after-dinner mints. Medline Plus.