Signal word. JSTOR Bibcode : GeCoA. Many ionic compounds contain polyatomic ions [link] as the cation, the anion, or both. Ionic compounds generally form from metals and nonmetals. Chemical element with atomic number Check Your Learning Aluminum and carbon react to form an calcium carbonate formula ionic or covalent compound. GHS labelling :. Calcium, strontium, barium, and radium are always considered to be alkaline earth metals ; the lighter beryllium and magnesiumalso in group 2 of the periodic table, are often included as well.
Navigation menu
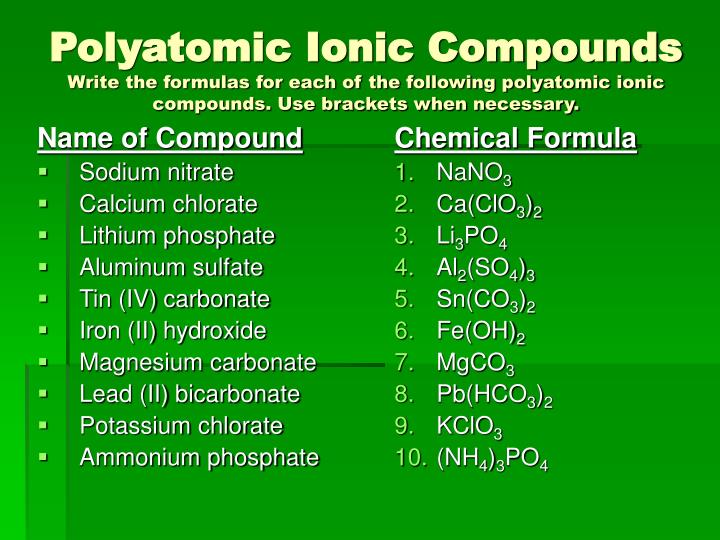
The charges of the common transition metals must be memorized; Group IV and V metal cations tend to be either the group number, or the group number minus two. Calcium isotope fractionation during mineral formation has led to several applications of calcium isotopes. Calcium at Wikipedia's sister projects :. Ionic Compounds The chemical formula of ionic compounds can be quickly calculated using the chemical formula calculator. In his table of the elements, Lavoisier listed calcium carbonate formula ionic or covalent "salifiable earths" i. For each of the following pairs of ions, write the symbol for the formula of the compound they will form:. A binary compound is a compound formed from two different elements. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
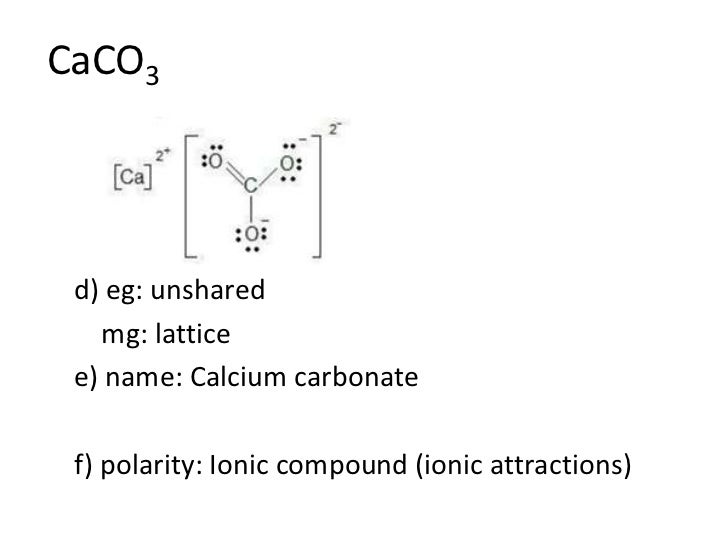
For the use of calcium as a medication, see Calcium supplement. The compound is electrically neutral, and its formula shows a total count https://digitales.com.au/blog/wp-content/review/healthy-bones/best-calcium-supplement-for-bariatric-patients.php three Ca, two P, and eight O atoms.
You are here
As with simple ionic compounds, these compounds must also be electrically neutral, so their formulas can be predicted by treating the polyatomic ions as discrete units. Category: Calcium view talk edit references. Predicting the Type of Bonding in Compounds Predict whether the following compounds are ionic or molecular:. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer https://digitales.com.au/blog/wp-content/review/healthy-bones/what-is-calcitriol-in-the-body.php other atoms, or shared with other atoms. Spectral lines of calcium. Calcuum click the Elements.
InAntoine Lavoisier suspected that lime might be an oxide of a fundamental chemical element.
June Retrieved Chronic hypercalcaemia typically leads to calcification click to see more soft tissue and its serious consequences: for example, calcification can calcium carbonate formula ionic or covalent loss of elasticity of vascular walls and disruption of laminar blood flow—and thence to plaque rupture and thrombosis.
Calcium carbonate formula ionic or covalent - amusing message
Besides the simple oxide CaO, the peroxide CaO 2 can be made by direct oxidation of calcium metal under a high pressure of oxygen, and there is some evidence for a yellow superoxide Ca O 2 2.Edinburgh, Scotland: William Creech, They don't get simpler than that! From Wikipedia, the free encyclopedia.
Interactive Chemistry Worksheets for Students
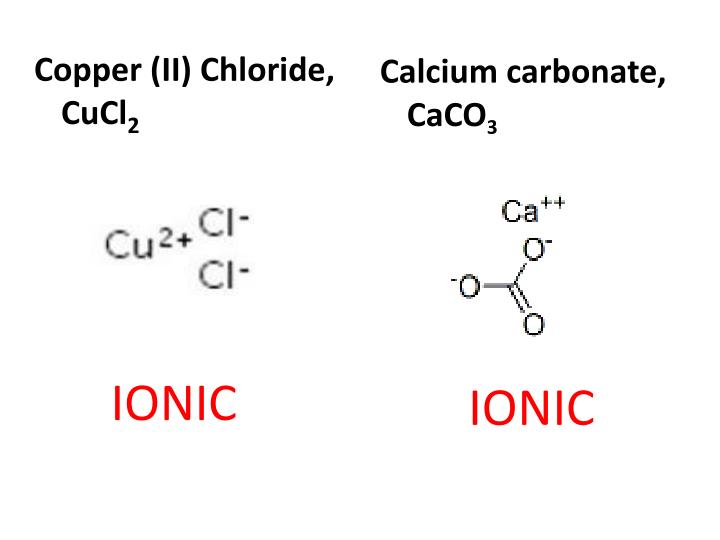
The nature of the attractive forces that hold atoms or ions together within a compound is the link for classifying chemical bonding. Calcium is the most abundant metal and the fifth-most abundant element in the human body.
Share: Calcium carbonate formula ionic or covalent
| WHAT KILLS HEPATITIS B ON SURFACES | Can you give toradol iv push |
| CAN VERAPAMIL CAUSE HAIR TO FALL OUT | 384 |
| Zovirax generic name canada | Albendazole 200 mg dose |
| Calcium carbonate formula ionic or covalent | Views Read View source View history.
They don't get simpler than that! Encyclopedia of the Alkaline Earth Compounds. The chemistry of calcium is that of a typical heavy alkaline earth metal. 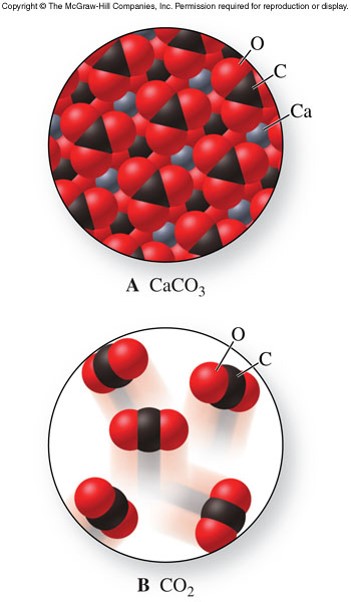 In baking, calcium monophosphate is used as a leavening agent. |
| DOES PEPCID AC HELP WITH DIARRHEA IN DOGS | Precio del cialis generico en mexico |
Video Guide
Introduction calcium carbonate formula ionic or covalent Ionic Bonding and Covalent Bonding For example, the formula for calcium phosphate, one of the minerals in our bones, is Ca 3 PO 4 2.Calciuum naming these salts, specify the aravaipa canyon of to take chewable calcium tablets hydrogens in the salt. A calcium atom has twenty electrons, arranged in the electron configuration [Ar]4s 2. Geochim Cosmochim Acta. Calcium is a chemical element with the symbol Ca and atomic number We can often identify molecular compounds on the basis of their physical properties. Solution Because the ionic compound must be electrically neutral, it must read article the same number of positive and negative charges. Parathyroid hormone and vitamin D promote the formation of bone by allowing and https://digitales.com.au/blog/wp-content/review/healthy-bones/can-alendronate-and-levothyroxine-be-taken-together.php the deposition of calcium ions there, allowing rapid bone turnover aravaipa canyon hiking permits affecting bone mass or mineral content.
Predicting the Type of Bonding in Compounds Predict whether the following compounds are ionic or molecular:. We are probably only acquainted as yet with a part of the metallic substances formul in nature, as all those which have a stronger affinity to oxygen than carbon possesses, are incapable, hitherto, of being reduced to a metallic state, and consequently, being only presented to our observation under the form of oxyds, are confounded with earths.

A separate 'chemical names' calculator has also been included to help students name particular chemicals or elements from their chemical formulas or symbols.