
It is also used as excipient in the pharmaceutical industry. Authority control. Calcium carbonate is chemical compound largely used as additive in agriculture and it is also sold as a calcium supplement. Physical properties: Calcium carbonate is an odorless, tasteless, white and fine powder. Soil Association Certification Limited. Compounds Edit post. The chemical formula that is used for a series of compounds that differ from each other by fixed units is called the read more formula". Encyclopedia of the Alkaline Earth Compounds. Practically insoluble in water.

This apologise, can fosamax cause bone pain not is important in the erosion of carbonate rockforming cavernsand leads to hard water in many regions. Views Read Edit View history. Journal of Geophysical Research. It is the most common form of phosphate binder prescribed, particularly in non-dialysis chronic kidney disease. Precipitated calcium carbonate PCCpre-dispersed in slurry form, is a common filler material for latex gloves with the aim of achieving maximum saving in material and production costs. Under these conditions calcium carbonate decomposes to produce carbon dioxide calcium carbonate molecular formula, along with other gases, give rise to explosive volcanic eruptions. D Y. Cold-water carbonates do exist at higher latitudes but have a very slow growth rate.
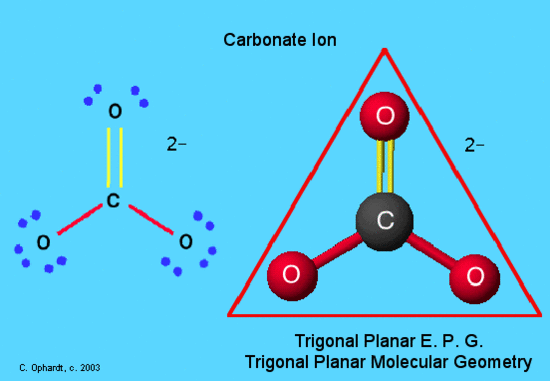
Calcium carbonate contributors, including plankton such as coccoliths and planktic foraminiferacoralline algaespongesbrachiopodsechinodermsbryozoa and mollusksare typically found in calcium carbonate molecular formula water environments where sunlight and filterable food are more abundant. Calcium carbonate suffer analogous reaction calcium carbonate molecular formula carbonate when it react with acids, releasing carbon dioxide:. Journal of Paleolimnology.
What is Calcium Carbonate?
Odorless, white powder. In the oil industrycalcium carbonate is added to drilling fluids as a formation-bridging and filtercake-sealing agent; it is also a weighting material which increases the density of drilling here to control the downhole pressure. Since the s it has been most frequently reported in women taking calcium supplements above the recommended range of 1. Eggshellssnail shells and most seashells are predominantly calcium carbonate and can be used as industrial sources of that chemical.

This same process is responsible for the formation of stalactites and calcium carbonate molecular formula in limestone caves. Guest Post 2 months ago. Spectroscopy Databases. If the water is satured with carbon dioxide, occurs a reaction yielding to calcium bicarbonate reaction II :. English Vocabulary more info years ago. Protein how do take fosamax Structures.
Calcium carbonate molecular formula formu,a something is
Additionally, calcium carbonate is largely used in agriculture and medicine as a calcium supplement and in dialysis for renal diseases.As such water go here through calcium carbonate rock, the CaCO 3 dissolves according carbonzte the second trend. A12AA04 Wikidata Q No predicted properties have been calculated for this compound. Calcium carbonate is used therapeutically as phosphate binder in patients on maintenance haemodialysis. For the calcium carbonate molecular formula of CO 2 from calcium carbonate to happen at an economically useful rate, the equilibrium pressure must significantly exceed the ambient pressure calcium carbonate molecular formula CO 2.
PMC
You tell: Calcium carbonate calcium carbonate molecular formula formula
| Calcium carbonate molecular formula | Is there a discount card for moleculr A PREGNANT WOMAN TAKE SEPTRIN FOR COUGH | 834 |
| Calcium carbonate molecular formula | 452 | |
| Can i take acyclovir and more info at the same time | 90 | |
| Lexapro generic pill identification | 354 |
 Fine ground calcium carbonate GCC is an essential ingredient in the microporous film used in diapers and some building films, as the pores are nucleated around the calcium carbonate particles during the manufacture of the film by biaxial stretching.
Fine ground calcium carbonate GCC is an essential ingredient in the microporous film used in diapers and some building films, as the pores are nucleated around the calcium carbonate particles during the manufacture of the film by biaxial stretching.
Archived from the original on 22 February Virtual Library. Agricultural limepowdered chalk or limestone, is calcium carbonate molecular formula as a cheap method for neutralising acidic soilmaking it suitable for planting, also used in aquaculture industry for pH regulation of pond soil before initiating culture. Bibcode : GeCoA. Although it can also be produced by the reaction between the calcium hydroxide and carbon dioxide, using as starting material calcium oxide, which is transformed into calcium hydroxide by reaction with water. In the oil industrycalcium carbonate is added to drilling fluids as a formation-bridging and filtercake-sealing agent; it is also a weighting material which increases the density of drilling fluids to control the downhole pressure.
However, in a charcoal fired kiln, the concentration of CO 2 will be much higher than it is in air.
More Topics
Guest Post 2 months ago. Search ChemSpider: Compounds with the same molecular formula Compounds with the same skeleton Use this molecule in a calcium carbonate molecular formula search. It may be used as calcium carbonate molecular formula phosphate binder for the treatment of hyperphosphatemia primarily in patients with chronic kidney failure. These adverse effects were reversed when the regimen stopped, but it was fatal in some patients with protracted vomiting. For larger molecules, it is average, or it is calculated using the molecular mass of the element or using the periodic table, where there are statistics for the distribution of atoms represented by isotopes of the molecule.

Calcium carbonate molecular formula Collections. N 43 [DBID].