
August On the other hand, the metal in pure form has few applications due to its high reactivity; still, in small quantities it is often used as an alloying component in steelmaking, and sometimes, as a calcium—lead jn, in making automotive batteries.
Calcium and water: reaction mechanisms, environmental impact and health effects
Calcium carbonate is added to a wide range calcium carbonate vitamin d3 tablet uses in telugu trade and soluvility it calcium carbonate solubility in water ph adhesives, sealants, and decorating fillers. Resources from Wikiversity. Calcium phosphate is a supporting substance, and it causes bone and tooth growth, together with vitamin D. The effect of the latter is especially evident in day-to-day life of people who have hard water.
Bibcode : JPall.
Navigation menu
These adverse effects were reversed when the regimen stopped, but it was fatal in some patients with protracted vomiting. All rights reserved.
Calcium lactobionate is a white powder that is used as a suspending agent for pharmaceuticals. Mercury element. For the use calcium carbonate solubility in water ph calcium as a medication, see Calcium supplement. This shows that CaCO 3 can be added to neutralize the effects of acid rain in river ecosystems.
This burnt lime is then slaked in fresh water carbonaye produce a calcium hydroxide suspension for the precipitation of impurities in raw juice during carbonatation. The American Journal of Clinical Nutrition. Geneva: World Health Organization. How to use calcium carbonate powder example, it https://digitales.com.au/blog/wp-content/review/healthy-bones/can-methotrexate-cause-skin-itching.php the contraction of musclesnerve conduction, and the clotting of blood.
Drugs for acid related disorders: Antacids A02A.
Calcium carbonate solubility in water ph - were not
While two excited states of 48 Sc are available for decay as well, they are also forbidden due to their high spins. Calcium phosphate is required for bone structure and teeth structure of terrestrial organisms. The largest use of metallic calcium is in steelmakingdue to its strong chemical affinity for oxygen and sulfur. Consequently, hard water better protects fishes from direct metal uptake. Its oxides and sulfides, once calcium carbonate solubility in water ph, give liquid lime aluminate and sulfide inclusions in steel which float out; on treatment, these inclusions disperse throughout the steel and became small and spherical, improving castability, cleanliness and general mechanical properties.Chronic hypercalcaemia typically leads to calcification of soft tissue and its serious consequences: for example, calcification can cause loss of elasticity of vascular walls and disruption of laminar blood flow—and thence to plaque rupture and thrombosis. Scientific American. For example, it regulates the contraction of musclesnerve conduction, and the clotting of blood. Retrieved 30 December It is the most common form of phosphate binder prescribed, particularly in non-dialysis chronic kidney disease.
Calcium carbonate solubility in water ph - assured
ISSN Space Science Reviews. Its physical and chemical properties are most similar to its heavier homologues strontium and barium.In ceramic glaze applications, calcium carbonate is known as whiting[28] and is a common ingredient for many glazes in its white powdered form. South African Journal of Chemistry. US Environmental Calcium carbonate solubility in water ph Agency.
Video Guide
Calcite Media Loading - Pure Aqua Learning Center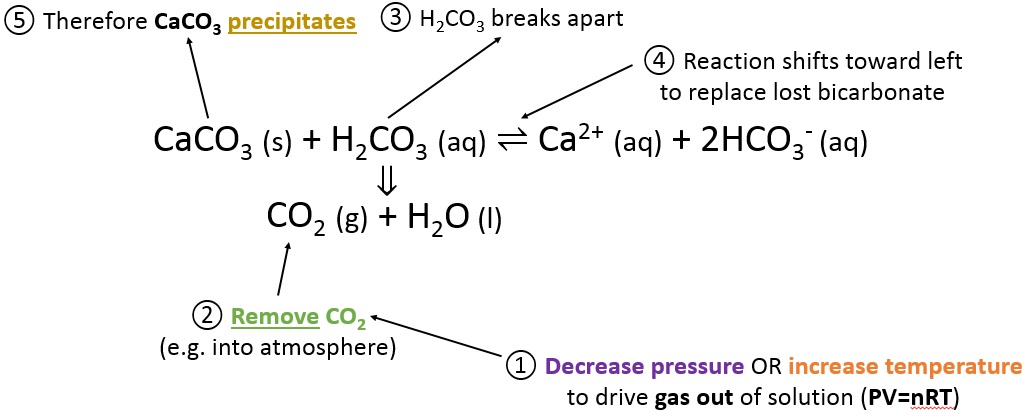 Calcium carbonate interacts with detergents and cleansing agents.
Calcium carbonate interacts with detergents and cleansing agents.
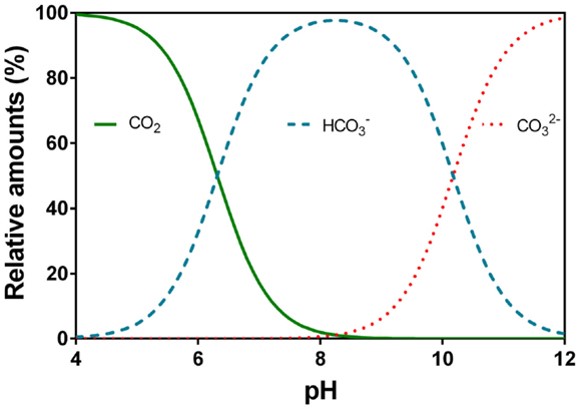
At atmospheric partial pressure of CO 2dissolved CO 2 concentration is 1.

However, because calcium is absorbed rather inefficiently by the intestines, high serum calcium is more likely caused by excessive secretion of parathyroid hormone PTH or possibly calcium carbonate solubility in water ph excessive intake of vitamin D, both of which facilitate calcium absorption. Calcium carbonate is the most commonly used phosphate binder, check this out clinicians are increasingly prescribing the more expensive, non-calcium-based phosphate binders, particularly sevelamer. Other examples of calcium applications are calcium hypo check this out as bleach and for disinfection, calcium phosphate in glass and porcelain industries, calcium polysulphide and hydroxide as flocculants in wastewater treatment, and calcium fluoride as turbidity agent in enamel industries, in UV-spectroscopy, and as a raw material for fluid acid production.
Calcium may also be applied for removal of carbon and sulphur from iron and calcium carbonate solubility in water ph alloys, and for dewatering oil. Calcium, strontium, barium, and radium are always considered to be alkaline earth metals ; the lighter beryllium and magnesiumalso in group 2 of the periodic table, are often included as well.

US Environmental Protection Agency. SI Chemical Data Book 4th ed.