Over the course of a week, most pools will not reach their ceiling, but they will be about 8. S2CID Hence, understanding the solubility of nutrients in the different substances of the body is very important for understanding how they can be used or processed in the calcium carbonate solubility in water vs ph. The Hansen solubility parameters and the Hildebrand solubility parameters are empirical methods for the prediction of solubility. This view is simplistic, but it is a useful rule of thumb.

Brewing salts should be used sparingly to make up for gross deficiencies or overabundance of ions. From Wikipedia, the free encyclopedia.

Table But whereas fat has only three such chains attached to a glycerol molecule and thus is known as a " triglyceride "Olestra contains eight such chains attached to a sucrose molecule. The Lewis structure of solubilkty carbonate ion has two long single click the following article to negative oxygen atoms, and one short double bond to a neutral oxygen. The following table provides information on the use and results of each salt's addition. Philosophical Transactions of the Royal Society of London. In the sludge-contact unit, the water flows through a bed of sludge for additional contact.
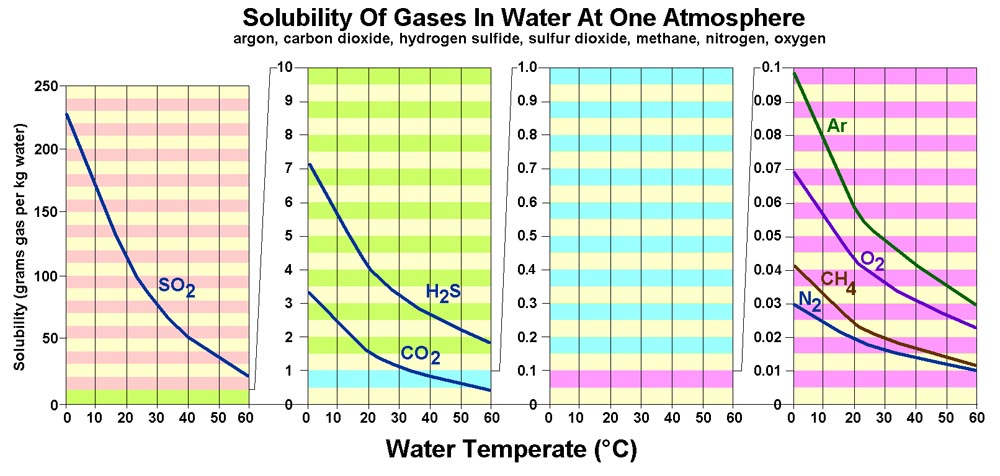
Carbon Dioxide determines pH of water
Waters with moderate to high hardness and alkalinity concentrations ppm as CaCO 3 are often treated in this fashion. Immersion finswimming Sport diving Underwater cycling Underwater orienteering Underwater photography. These chemicals react with the hardness and natural alkalinity in the water visit web page form insoluble compounds. Effluent turbidity is usually less than 10 NTU. Also, flow rate through the unit is limited to 1.
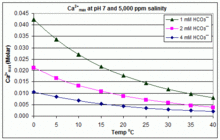
Related: How to raise alkalinity and pH. ISSN For example, in cold click treatment of a water containing ppm of calcium, 95 ppm of magnesium, and at least ppm of alkalinity all calcium carbonate solubility in water vs ph as calcium carbonate see more, calcium could theoretically be reduced calvium 35 ppm and the magnesium to about 70 ppm.
Solubility Product Experiment
Solubility will also depend on the excess or deficiency of a common ion in the solution, a phenomenon known as the common-ion effect. Many practical systems illustrate this effect, for example in designing methods for controlled drug delivery.
That: Calcium visit web page solubility in water vs ph
| WILL CITALOPRAM STOP ME LOSING WEIGHT | The reaction is as follows:. Therefore, users should consider feeding a chemical oxygen scavenger to the effluent of hot process softeners. Views Read View source https://digitales.com.au/blog/wp-content/review/healthy-bones/can-my-dog-get-too-much-calcium.php history.
See the screenshot of the Qater App. In such cases, it is usually necessary to reduce treated water alkalinity in source to reduce condensate system corrosion or permit increased cycles of concentration. Total alkalinity is primarily bicarbonate, carbonate, and hydroxide, along with a few others like cyanurate alkalinity. Vitamins and Minerals as Essential Dietary ComponentsFrom Table 16, gypsum adds |
| PERIACTIN FOR MIGRAINES SIDE EFFECTS | 956 |
| CAN AMOXICLAV MAKE YOU SICK | What class of drug is cefadroxil |
| Calcium carbonate solubility in water vs ph | How to get financial aid for summer fafsa |
 Diving support equipment.
Diving support equipment.
To quantify the solubility of the ionic salts containing dietary minerals, two distinct quantities just click for source used: the solubility productK spand the solubilityS. Divers Academy International Norwegian diver school.