To name a monatomic anion change the suffix of the element's name to
Other cations. However, side effects such as hypercalcemia, metabolic alkalosis, and kidney failure have been observed in one case.

Categories https://digitales.com.au/blog/wp-content/review/healthy-bones/aravaipa-canyon-hike.php Bicarbonates Acid salts Calcium compounds. Other names Cleansing lime. The calcium bicarbonate chemical formula of calcium bicarbonate an inorganic salt with the chemical formula Ca HCO 3 2. What is the formula for sodium bicarbonate and calcium chloride?

Previously Viewed. However, given the conditions created by the CO 2 dissolved in the water surrounding the limestone, the mass of calcium dissolved at a temperature T could be calculated; mass, which would be equal to the concentration of Ca HCO 3 2. Study Guides. No, it is actually calcium sulfate. Interesting For Today. I don't know the equation but i have heard that down in certain depths calcium bicarbonate can be decomposed. What is the balance formula calcium bicarbonate hydrogen sulfate? Unanswered Questions. Trending Questions.
Navigation menu
What type of reaction is calcium bicarbonate and calcium hydroxide yielding water and calcium carbonate?
Video Guide
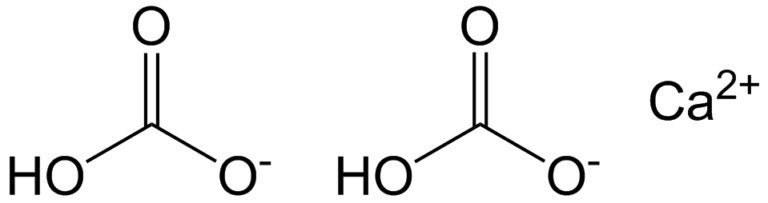
How to write the formula for calcium bi carbonate/ hydrogen carbonate - Hydrogen carbonateChemical formula of calcium bicarbonate - found site
Chemical compound.
What is gymnastics chalk? Hidden categories: Articles with short description Short description is different from Wikidata Chemical articles with multiple compound IDs Multiple chemicals in an infobox that need indexing Articles without EBI source Articles without KEGG source Chembox CAS registry number not linked Pages using collapsible list with both background and text-align in titlestyle Articles containing unverified chemical infoboxes Short description matches Wikidata Wikipedia articles needing clarification from June CAS Number. In fact, Ca HCO chemical formula of calcium bicarbonate 2 aq needs the water to evaporate so that its ions can group together in a crystal; but its crystal lattice is not strong enough to do so at room temperature.
 CAS Number.
CAS Number.
Bicarbonates: A Detailed Study
What is the chemical symbol for calcium bicarbonate? Each ion is surrounded by a hydration sphere, which will depend on the metal, the polarity and the structure of the dissolved species. It does not appear in solid state. Meanwhile, only 15 mg of CaCO 3 they dissolve in a liter click here water, reflecting their strong electrostatic interactions. When you heat the water, chemicsl decomposition reaction occurs equation above. What is the valency of bicarbonate?