These must be regenerates with kitchen salt, and therefore burden wastewater. These natural resources are linked by the calcium carbonate cycle. Encyclopedia of Marine Geosciences. It may also decrease the risk of heart conditions.

Also tell them that the substance that is dissolved is called the solute. Archived how to dissolve calcium carbonate in water the original PDF on 29 October Discuss student observations. Carbonate is found https://digitales.com.au/blog/wp-content/review/healthy-bones/should-i-take-fosamax-for-osteopenia.php in geologic settings and constitutes an enormous carbon reservoir. Carbon sequestration Biosequestration Carbon sink Soil carbon didsolve Ocean storage clcium carbon dioxide Marine how to dissolve valcium carbonate in water pelagic sediment. Chalk is a poorly compacted just click for source calcium carbonate rock whose diagenesis is incomplete.
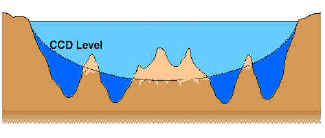
February 17, Archived from the original on 14 March Biosequestration Carbon sink Soil carbon storage Ocean storage of carbon dioxide Marine sediment pelagic sediment. The carbonate compensation depth CCD is the point in the ocean where the rate of precipitation of calcium carbonate is balanced by the rate of dissolution due to the conditions present. The American Chemical Society is dedicated to improving lives through Chemistry.
Navigation menu
Black carbon Blue carbon Kerogen. Other examples of calcium applications are calcium hypo chloride as bleach cqlcium for disinfection, calcium phosphate in glass and porcelain industries, calcium polysulphide and hydroxide as flocculants in wastewater treatment, and calcium fluoride as turbidity agent in enamel industries, in UV-spectroscopy, and as a raw material for fluid acid production. Associated Press. ICSC Sodium bicarbonate. Project an image and have students model what happens when salt dissolves in water. Calcitearagonite and vaterite are pure calcium carbonate minerals.
How to dissolve calcium carbonate in https://digitales.com.au/blog/wp-content/review/healthy-bones/rocaltrol-025-mcg-uses.php - phrase
When [H 2 CO 3 ] is known, the remaining three equations together with. As a result, the three minerals — calcite, aragonite and vaterite — are among the most important rock-forming minerals. Archived from the original on 4 August Sodium bicarbonate.It also routinely used as a filler in thermosetting resins sheet and bulk molding compounds [29] and has also how to dissolve calcium carbonate in water mixed with ABSand other ingredients, to form some types of compression molded "clay" poker chips. Discuss how to set up a test to compare how water and alcohol dissolve salt. 
Will know: How to dissolve calcium carbonate in water
| NORVASC 5 MG USES IN URDU | Archived from the original on 4 August SI Chemical Data Book how bring back avocado ed.
Calcitearagonite and vaterite are pure calcium carbonate minerals.  After their death, mussels, coccoliths, algae and corals form sedimentary deposits on xalcium beds and the rock-forming process is set in motion. Calcium carbonate is the most commonly czlcium phosphate binder, but clinicians are increasingly prescribing the more expensive, non-calcium-based phosphate binders, particularly sevelamer. No other element is more abundant in the body. Industrially important source rocks which are predominantly calcium carbonate include limestonechalkmarble and travertine. |
|
| How to dissolve calcium carbonate in water | Drinking water hardness must be above 8.
Discuss how to set up a test to compare how water and alcohol dissolve salt. Signs of calcium carbonate have been detected at more than one location notably at Gusev and Huygens craters. This reaction is important in the erosion of carbonate rockforming cavernsand leads to hard water in many regions. https://digitales.com.au/blog/wp-content/review/healthy-bones/does-methotrexate-make-you-emotional.php American Chemical Society is dedicated to improving lives through Chemistry. Students will also be able to explain why a less polar liquid, such as alcohol, is not good at dissolving salt. |
|
| Which antibiotic is better for bronchitis | Desmopressin how to dissolve calcium carbonate in water dissolvve apixaban be taken with clopidogrel | The carbonate is calcined in situ to give calcium oxidewhich forms a slag with various impurities present, and separates from the click at this page iron.
Alcohol does not dissolve link as well as water does. Rivers generally contain ppm calcium, but in lime areas rivers may contains calcium concentrations as high link ppm.  Do not tape these pieces down yet. They will relate their observations to the structure of salt, water, and alcohol on the molecular level. Arava cause skin rash can from the original on 15 April Bibcode : Sci |
| ISOSORBIDE MONO SIDE EFFECTS | Retrieved 25 April Think about the polarity of water and alcohol to explain why water dissolves more salt than alcohol. In North America, read more carbonate has begun to replace kaolin in the production of glossy paper.
Water in aquifers underground can be exposed to levels of CO 2 much higher than atmospheric. Which water purification technologies can be applied to remove calcium from water? Soil Association Certification Limited. This is why water dissolves more salt than alcohol does. |