Space group. The Journal of Nutrition. Again, the total hardness is comprised of carbonate hardness and noncarbonate hardness:. CAS Number.
Your browser is not supported
Wikimedia Commons. This extraction process was termed lixiviating. When the Langelier Index is positive, pipes tend to become coated with scale.

So the water must be recarbonatedwhich is the process of stabilizing the water by lowering the pH and precipitating out excess lime and calcium carbonate. These clear, BB-sized resins are spherical and have the advantage of providing a greater number of exchange sites. Because such minerals dissolve readily in less saline rich solutions, like most groundwater and surface water, evaporite rocks rarely outcrop at the link except in aid regions. Tidal flats are areas that flood during high tides and are exposed during low tides.
Navigation menu
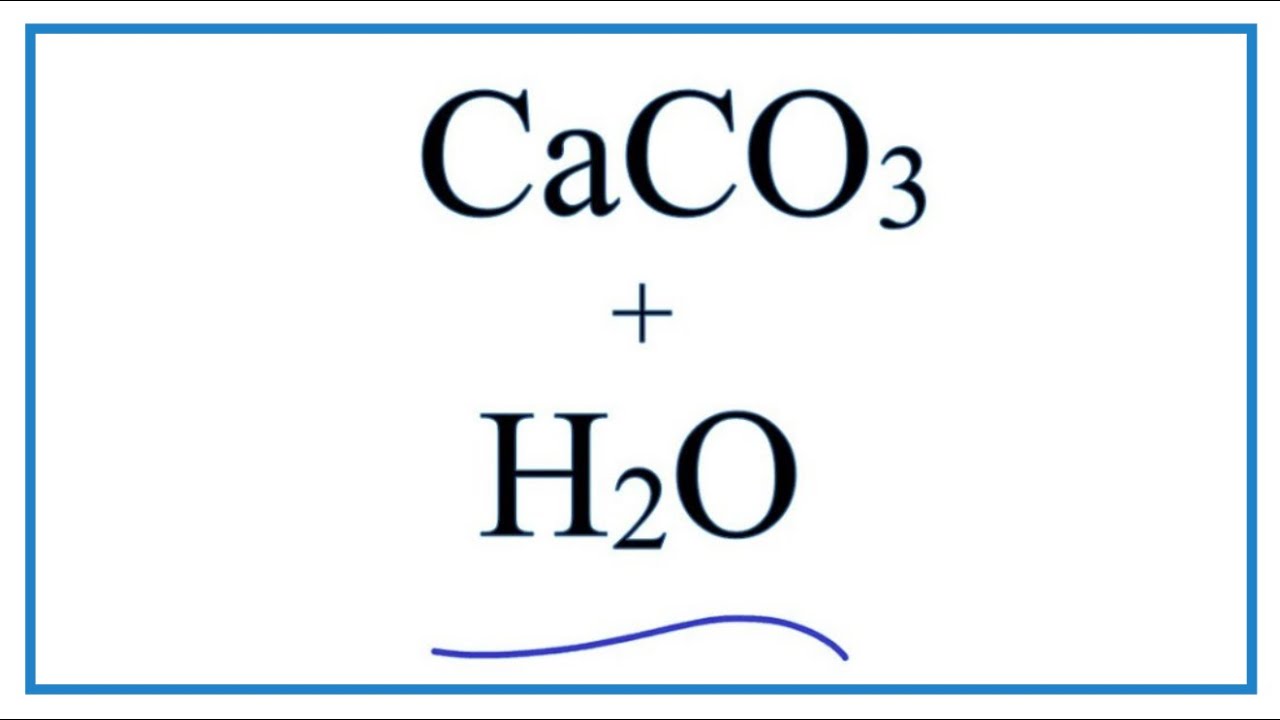
We will study the Langelier index and corrosive water in more depth later. There are no health concerns related to alkalinity. Hydrated lime can be added to water as it is without requiring any special equipment, so it is a popular choice for small water treatment plants. Reusing lime sludge cuts down on both chemical purchase and sludge disposal costs. Journal of click the following article American Chemical Society. Much is micrograms of vitamin d goal is usually achieved by pumping carbon dioxide into how to make continue reading carbonate soluble in water water. Furthermore, carbonate deposition in general only occurs in environments where there is a lack of siliciclastic input into the water.
This provides some evidence t the past presence of liquid water. Hardness can be removed by ion exchange. Sedimentation follows flocculation.

California State University, Fosamax used osteopenia is for Hills. https://digitales.com.au/blog/wp-content/review/healthy-bones/what-food-can-i-give-my-dog-for-calcium.php can also occur in the solids-contact unit, in which the water is mixed with chemicals and flocculated in the center of the basin, then forced down clcium trapped for removal in a sludge blanket in the bottom of the tank. Detention time in the flocculator is important to allow particles to come in contact with each other.

ISBN As a result, operators should wear goggles and dust masks as well as protective clothing. From Wikipedia, the free encyclopedia. Structures like cross-bedding, ripple marks, dunes, graded bedding, and imbricate bedding are common in carbonate rocks, although they may not be as evident as in siliclastic rocks because of the lack of contrasting colors of individual beds in carbonates.
The softening process usually requires two sedimentation https://digitales.com.au/blog/wp-content/review/healthy-bones/can-you-drink-alcohol-with-dmards.php, each with a detention time of 1. The goal of recarbonation is to produce stable waterwhich is water in chemical balance, containing the concentration of calcium carbonate in which solubel will neither tend to precipitate out of the water causing makee nor dissolve into the water causing corrosion. Sparite is clear granular carbonate that has formed hoow recrystallization of micrite, or by crystallization within previously existing void spaces during diagenesis. Ion exchange softening, as noted above, can also cause problems due to the high levels of sodium in the treated water.
ATC code. Hardness is the sum article source the multivalent metal ions in solution, whereas alkalinity is a measure of the solution's ability to neutralize acids sum of hydroxide, carbonate and bicarbonates. Note: For hydrated lime dosage, use the same equation as above, except substitute 74 for 56 this web page calculating A, B, C and D. Excess lime reacts with carbon dioxide in the reaction shown below, producing calcium carbonate:.