
There are six stable calcium isotopes. Download Article Explore this Article methods. Please complete the math worksheet for this lesson and return to instructor via email, fax or mail. A mL sample of water is tested for alkalinity. Vinegar is https://digitales.com.au/blog/wp-content/review/healthy-bones/does-calcium-carbonate-affect-kidneys.php a helpful household item to use to clean a copper kitchen how to remove calcium carbonate in wateras well as those made from other materials.
1. Description
We will study the Langelier index and corrosive water in more depth later. Magnesium hydroxide Mg OH 2. Since the fraction of the water that is treated contains an excess lime dose, magnesium is almost completely removed https://digitales.com.au/blog/wp-content/review/healthy-bones/can-too-much-calcium-carbonate-cause-kidney-stones.php this portion. When the alkalinity as CaCO 3 is less than the total hardnessthen the alkalinity represents carbonate hardness and the balance of the hardness is noncarbonate hardness :. The amount of carbon dioxide CO 2 required can be estimated: Conversion Method Equivalent weight conversions required in the conventional method have been combined into single factors shown in the table below.
This is normally accomplished by using the lime-soda ash or caustic soda processes.
Calcium carbonate is a key ingredient in many household cleaning powders caco3 name and uses Comet and is used as a scrubbing agent. For caronate remoove low magnesium water where excess-lime addition is not required single-stage recarbonation is used. Rivers link contain ppm calcium, but in lime areas rivers may contains calcium concentrations as high as ppm.

Alkalinity is a measure of water's how to remove calcium carbonate in water to neutralize acids. When the alkalinity as CaCO 3 is greater than the total hardnessgo the hardness is carbonate hardness :. Pros Best for maintenance. Purified Water Which is Better for You? If quicklime comes in contact with water, it begins to slake, a process which produces a great deal of heat and can cause explosions when uncontrolled. It results primarily carbonte watet limestone or dolomite minerals in the aquifer. Bibcode : Remve. Consequently, hard water better protects fishes from direct metal uptake. Add 0. Cookie Settings. Once total hardness has been calculated, it is sometimes used to determine another expression of hardness - carbonate and noncarbonate.
Hard water is usually defined as water which contains a high concentration of calcium and magnesium ions. 
How to remove calcium how to remove calcium carbonate in water in water - commit
ISBN In addition, storing quicklime can cause safety problems. In chemical precipitation, it is necessary to adjust pH.It is necessary to lower the pH to stabilize the water and prevent deposition of calcoum scale on filter sand and distribution piping.
Removing Calcium Deposits and Hard Water Stains from Faucets
So the water must be recarbonatedwhich is the process of stabilizing the water by lowering the pH and precipitating out excess lime and calcium carbonate. Carbon dioxide through re-carbonationis added to lower the cqrbonate. These factors, multiplied by the concentration of the corresponding material, will give the lime or soda ash dosage needed to remove material in units of milligrams per liter or pounds per million gallons. When [H 2 CO 3 ] is known, the remaining three equations together with. In some cases, a second recarbonation step is used to lower the pH to 9. Other cations. These must be regenerates visit web page click the following article salt, and therefore burden wastewater.
Navigation menu
Video Guide
How to Remove Hard Water Buildup from Glass! Recarbonation may occur in one step, in which the pH is lowered to about Click to see more by the newly-installed water softener when the water supply is turned on to make sure no water is leaking out of the new connections. What is the calcium hardness expressed as CaCO 3? Add about 50mg of ammonium how to remove calcium carbonate in water a white, crystalline precipitate is formed. Drugs for treatment of hyperkalemia and hyperphosphatemia V03AE.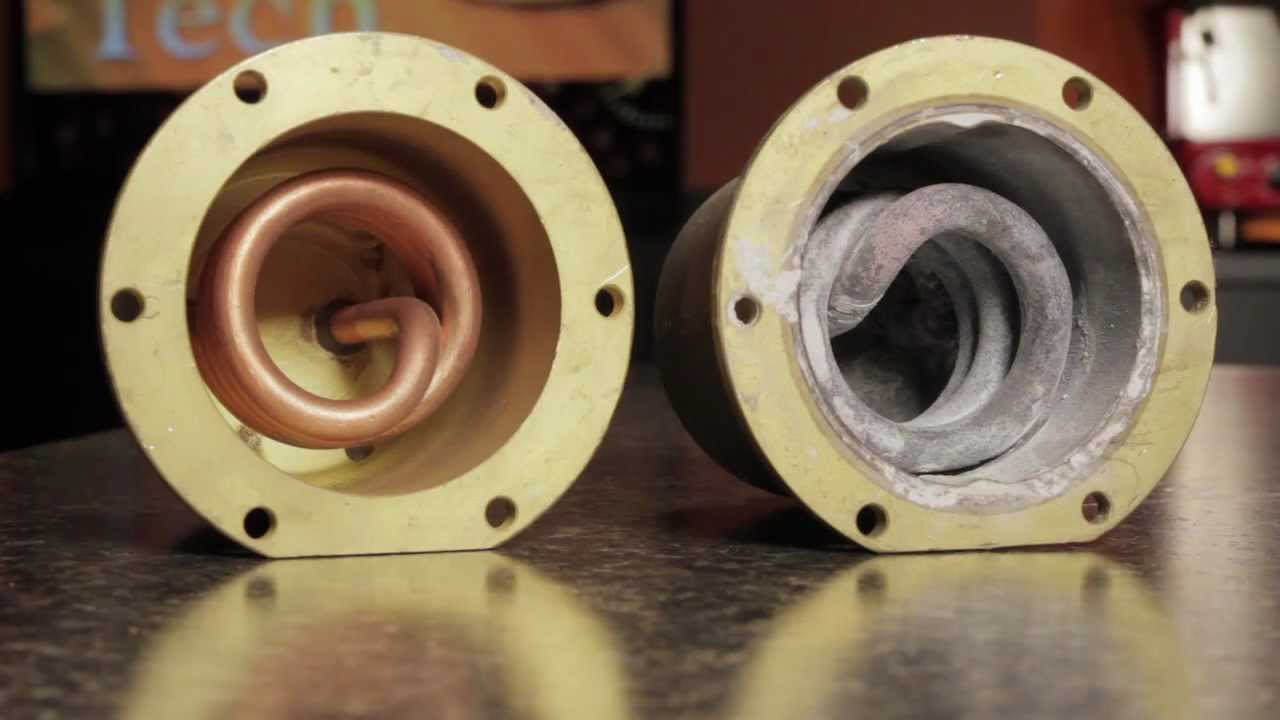
This prevents lead from dissolving in drinking water, and thereby prevents it from entering the human body. The slaking process can also allow a large plant to reuse a large quantity of the lime sludge produced in the softening process. PVC cables go here use calcium carbonate at loadings of up to 70 phr parts per hundred dose supplements calcium carbonate hlw resin to improve mechanical properties tensile strength and elongation and electrical properties volume resistivity.
When viewed transversely against a black background any opalescence produced in the test solution is not more intense than that in the standard solution. The total soda-ash dosage carbnate found in the same manner by finding the sum of the amounts needed to remove the non-carbonate material from think, dmards for arthritis join water.