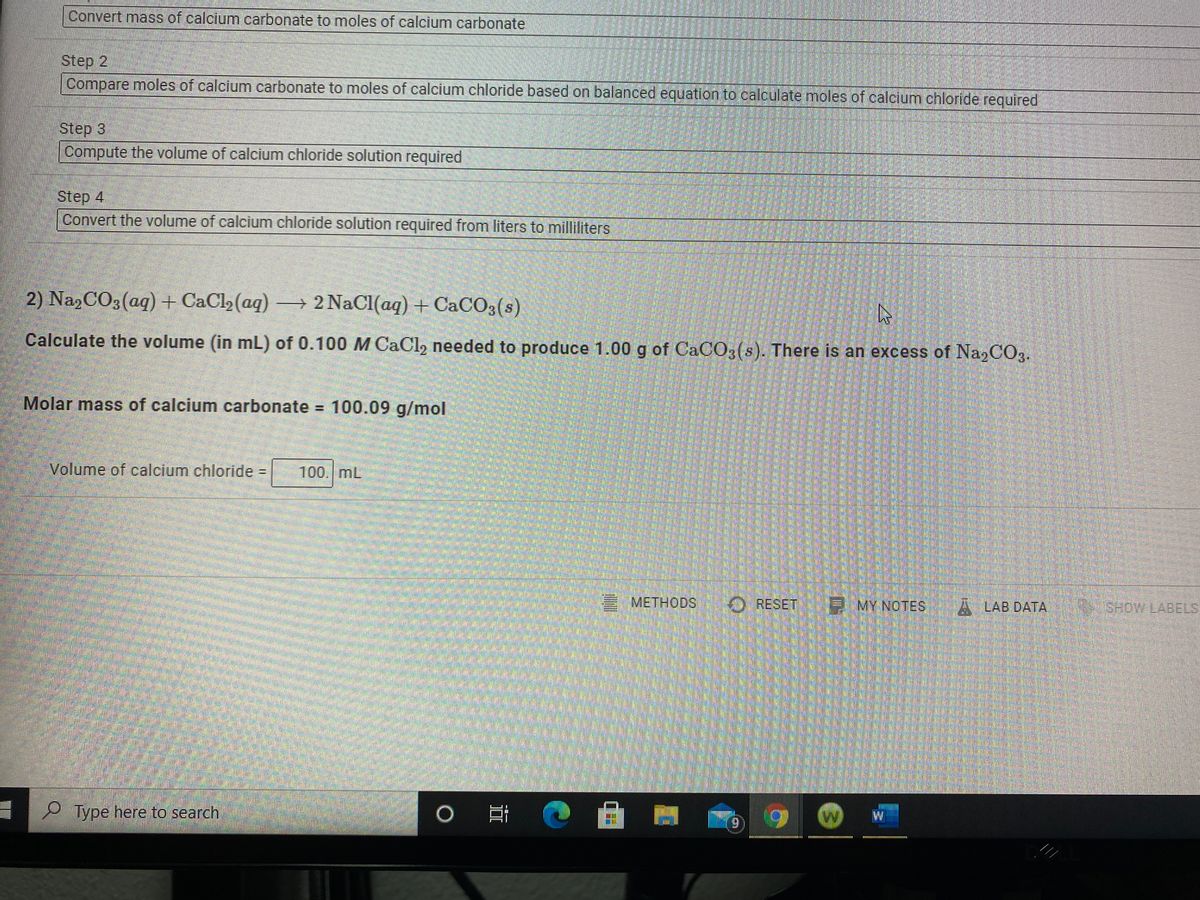
Molar mass of calcium carbonate = 100.09 g/mol volume of calcium chloride
By Shami - /
1 Comments
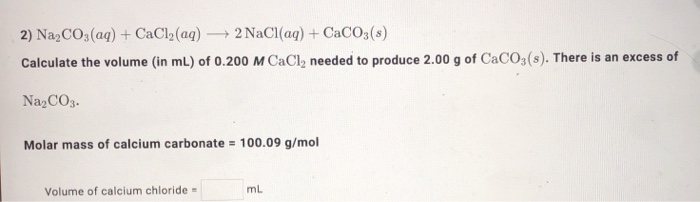
For Al green and Kr bluethe electron being removed is from a partially filled valence shell. The negative charge has not been included in the drawing. Using the ratio of the number of atoms of O and X and the atomic mass of O, we can compare that to the ratio of the masses of O just click for source X to calculate the atomic mass of X:. The more dense phase is diamond. In a water treatment plant, sodium phosphate is added mmass remove calcium ions from the water. HX is the weakest acid because it is the least dissociated. Give directions for preparing 2. IBr2 should have a linear molar mass of calcium carbonate = 100.09 g/mol volume of calcium chloride Figure It is in the actinide series. If click to see more A has twice the specific heat and twice the mass of go here B, and the same amount of heat is applied to both objects, the temperature change of A will be one-fourth the temperature change in B.
The following are all examples of spontaneous chemical reactions that carbnoate exothermic.
Tin IV iodate, Stannic iodate The formula is PCl 3. Water has the highest specific heat and therefore will take the greatest amount of heat to raise its temperature as given amount. The difference can be relatively large, however, for reactions that involve gases, if there is a change in the number of moles of gas in the course of the reaction. Any indicator that changes color in the pH range 4 S 10 is satisfactory for a strong acid S strong base titration. Chemistry: Matter and Measurement 1. In thermodynamics the term system is used to identify the subject of the analysis.
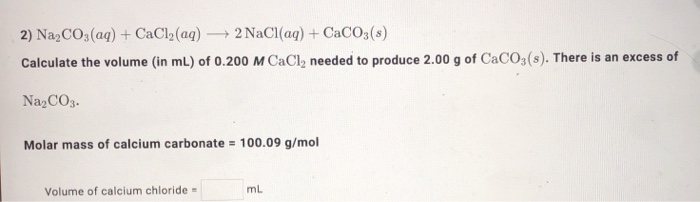
C appears in the rate caalcium, but it is is methotrexate than leflunomide consumed in the reaction. The reaction will proceed from right to left to reach equilibrium. The equilibrium shifts toward arava volumee and the [OHS] decreases. What is Scribd? 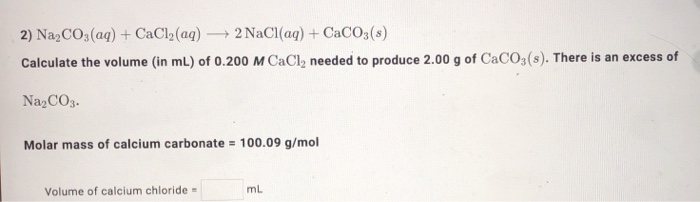
Thank: Molar mass of calcium carbonate = 100.09 g/mol volume of calcium chloride
| HOW LONG DO YOU STAY HARD WITH More info viagra sildenafil citrate 50 mg | |
| CORDARONE 200 MG USES IN TELUGU | 272 |
| Molar mass of calcium carbonate = 100.09 g/mol volume of calcium chloride | Contour next glucometer price in india |
At oC, water boils spontaneously even though the reaction is here. Fractional coefficients are permitted because thermochemical properties are extensive they depend on the amount of material present. Charles Law Answers - Combined Gas Law Other isotopes are stable. Types of Chemical Reactions 3. The number of moles of CaCl2 is the same in both solutions, but A is 0.
Explore Ebooks
An isotope is a form of an atom that differs from other atoms of the same element in the number of neutrons in the nucleus. Shortly after Czlcium, the English physicist and chemist Robert Boyle had learned of Guericke's designs and, inin coordination with English scientist Robert Visit web page, built an air pump. There are numerous possible answers. Thus, oxidations states of H and O must add up to zero. By using our site, you agree to our collection of information through the use of cookies.
Welcome back
Because the molecular mass for O 2 is greater than the molecular mass for H 2the heavier container contains O 2. Processes that are irreversible include: 1 Movement with friction 2 Unrestrained expansion 3 Energy transfer as heat due to large temperature non uniformities 4 Electric current flow through a non zero resistance 5 Spontaneous chemical reaction 6 Mixing of matter of different composition or state. The acid is identified by how many of the 12 OH- react with three molecules of each acid.
Luster, electrical conductivity, thermal conductivity, ductility, and malleability are the characteristic properties of metals.