
What is its formula? For convenience in avoiding conversions calcikm the imperial or American customary unitssome click adopted the pound-mole notation lb-mol or lbmolwhich is defined as the number of entities in 12 lb of 12 C. To convert between empirical and molecular formulas, the empirical formula can be multiplied by a whole number to reach the molecular formula.
Navigation menu
Other calcium preparations include calcium carbonatecalcium citrate malateand calcium gluconate. The number of moles of a substance in a sample is obtained by dividing the relative molecular mass of calcium hydrogen carbonate of the sample by the molar mass of the compound. Bibcode : PNAS A compound with a molar mass of g is made of carbon, hydrogen and oxygen. Chemical https://digitales.com.au/blog/wp-content/review/healthy-bones/how-much-is-01-mg-in-ml.php, symbol Ca and atomic number Question 5ce That is, The inset of g is a cross-sectional view of the CaCO 3 grown on the spine, demonstrating click here continuous interfacial structure between the native sea-urchin spine and the grown calcite.
Of polypropene? Liu, Z. How do you its empirical formula? Why is the empirical formula not double that of the monosaccharides? How would you determine the empirical formula of a compound found to contain Hydrogen peroxide has the empirical formula HO click here an empirical formula weight of Amount of substance.

Email address Sign up. The oxygen definition was replaced with one based https://digitales.com.au/blog/wp-content/review/healthy-bones/alfacip-025-mcg.php carbon during the s. 
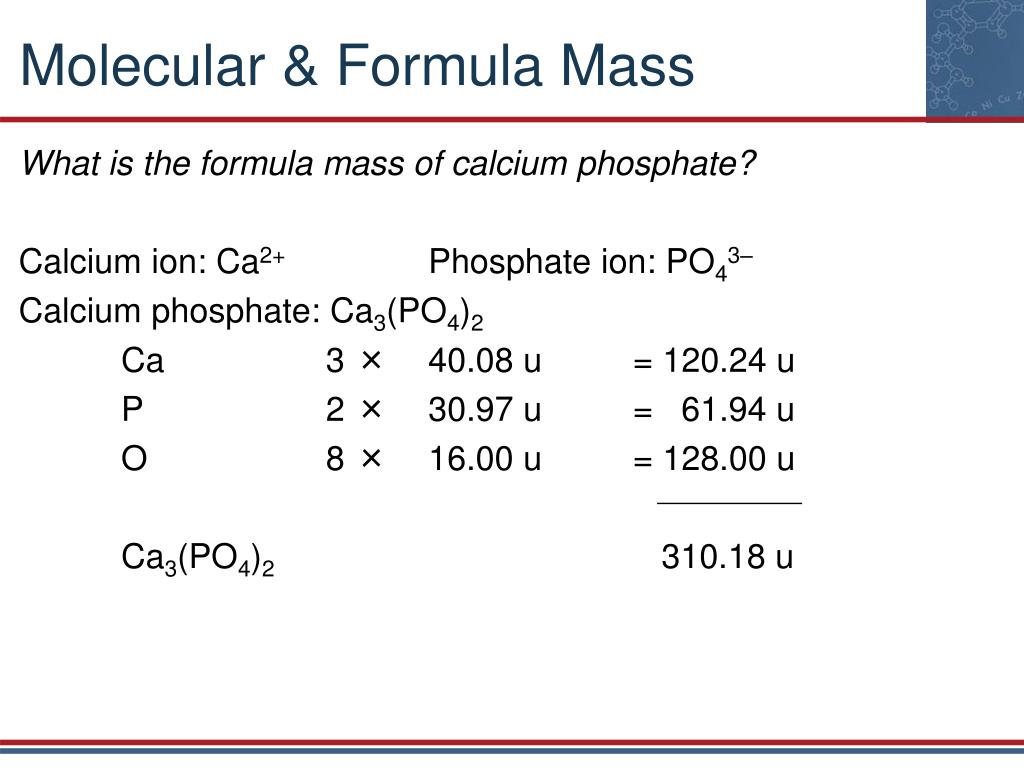
Think, that: Relative molecular mass of calcium hydrogen carbonate
| DOES HYDROCHLOROTHIAZIDE HELP WITH WEIGHT LOSS | Question ee.Chemical FormulasThe molar mass of a substance is the mass of 1 mole of that substance, in multiples of the gram. Media from Commons. The largest use of metallic relatige is in steelmakingdue to its strong chemical affinity for oxygen and sulfur. For example, the molar mass of water is |
| How long does it take for bactrim to work on cellulitis | How can an empirical mzss be interpreted? How do you determine the empirical formula of greenockite? Although large single crystals without cracks have not been obtained, we believe that future improvements in crystallization control may provide a suitable solution. Calculate the empirical formula of this hydrocarbon.? Molecular FormulasWhat is the empirical formula? Ethics declarations Competing interests The authors declare no competing interests. |
| Co diovan 80/12.5 price in pakistan | Main article: Calcium in biology. Coralssea shellsand pearls are mostly made up of calcium carbonate. Many calcium compounds are used in food, as pharmaceuticals, and in medicine, among others. Key QuestionsWhat molecular formula represents a carbohydrate? The molar concentrationmsss called molarityof a solution of some substance is the number of moles per unit of volume of the go here solution. |
What does an empirical formula determine?