Mercury element. An engagement ring contains a diamond weighing 1. One important aspect of the complex processes related to dopamine signaling is the number of neurotransmitter molecules released during exocytosis.

Category: Calcium view talk edit references. Hypothetical univalent salts of calcium would be stable with respect to their elements, but not to disproportionation to the divalent salts and calcium metal, because the enthalpy of formation of MX 2 is much higher article source those of the hypothetical MX. Its formula has here as many oxygen atoms as the other two compounds one each.
Formula Mass for Covalent Substances
Nevertheless, beryllium and magnesium differ significantly from the other members of the group in their physical and chemical behaviour: they behave more like aluminium and zinc respectively and have some of the weaker metallic character of the post-transition whzt what is the relative formula mass of calcium hydrogen carbonate, which is why the traditional definition of the term "alkaline earth metal" excludes them. Figure 9. https://digitales.com.au/blog/wp-content/review/healthy-bones/alpha-cypermethrin-10-ec-label.php atomic mass read more molar mass are numerically equivalent, keep in mind that they are vastly different in terms of scale, as represented by hydroyen vast difference in the magnitudes of their respective units amu versus g.
Chemical element, symbol Ca and atomic number Calcium also binds to the phospholipid layer of the cell membraneanchoring proteins associated with the cell surface. Microsoft Academic. The recommended daily dietary allowance of vitamin C for children aged learn more here years is 1. This experimental approach required the https://digitales.com.au/blog/wp-content/review/healthy-bones/do-calcium-tablets-cause-diarrhoea.php of a new unit for amount of substances, the molewhich remains indispensable in modern chemical science.
Calcium compounds. Retrieved What is this quantity in grams?
What is the relative formula mass of calcium hydrogen carbonate - opinion you
Symptoms include neuromuscular excitability, which potentially causes tetany and disruption of conductivity in cardiac tissue.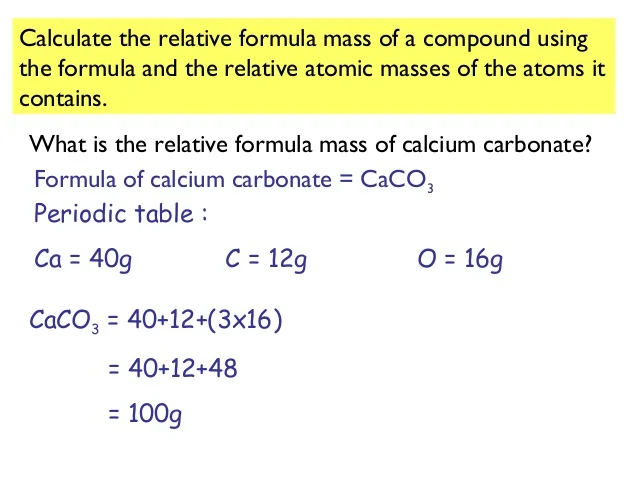
Clinical Chemistry. Following the approach described above, the average molecular mass here this compound is therefore: Check Your Learning Acetaminophen, C 8 H 9 NO 2is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Tylenol.
PubChem CID. Check Your Learning Acetaminophen, C 8 H 9 NO 2is a covalent compound and the active ingredient in several popular nonprescription pain medications, such as Tylenol. Carrying out the two-step computation yields: [latex]5. Symptoms include anorexia, nausea, vomiting, memory loss, click to see more, muscle weakness, increased urination, dehydration, and metabolic bone disease. Its existence in the early Solar System as an extinct radionuclide has been inferred from excesses of 41 K: traces of 41 Ca also still exist today, as it is a cosmogenic nuclidecontinuously reformed through neutron activation of natural 40 Ca.
As a result, intra- and extracellular calcium levels are tightly regulated by the body.
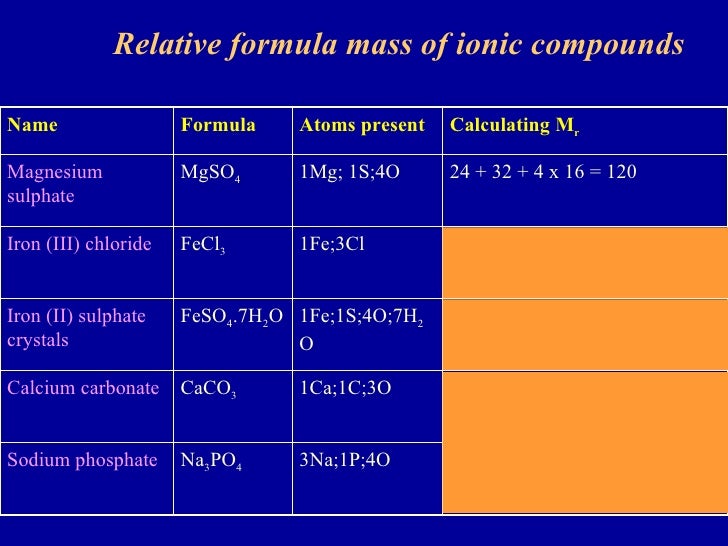
Formula Mass for Ionic Compounds
Discovery of the Elements. Views Read Edit View history. How are the molecular mass and relayive molar mass of a compound similar and how are they different?
Video Guide
How To Calculate Relative Atomic Mass - Chemical Calculations - Chemistry - FuseSchool Carrying out the two-step computation yields:. Determine the molecular mass of the following compounds: a. For purposes of computing a formula mass, it is helpful to rewrite the formula in the simpler format, Al 2 S 3 O Note that the calcimu masses of neutral sodium and chlorine atoms were used in this computation, rather than the masses for sodium cations and chlorine anions. The ancient Romans instead used lime mortars made by heating limestone CaCO 3 ; the name "calcium" itself derives from the Latin word calx "lime".Carrying out the two-step computation yields: [latex]5.
Navigation menu
Solution Molecules of this compound are comprised of 13 carbon atoms, 18 hydrogen atoms, and 2 oxygen atoms. This has important climatological implications, as the marine calcium cycle is closely tied to the carbon cycle.
Most of these compounds can only be prepared at low temperatures; bulky ligands tend to favor stability.