
HCl, or hydrochloric acid, is a strong acid that reacts with sodium carbonate.

Try the given examples, carbonaate type in your own problem and check your answer with the step-by-step explanations. Sodium Chloride solution and insoluble iron II hydroxide are produced. State the Question. This table can be used to help you work what is the word equation for calcium carbonate and hydrochloric the chemical formula of the reactants and products. We welcome your feedback, comments and questions about this site or page. Yes, we have written a word equation. Large Spill: Corrosive liquid.
Identifying Reactants and Products in Chemical Reactions
Answers: 2 on a question: Click at this page the reaction of sodium hydrogen carbonate and hydrochloric acid, describe the reactant appearance and identify the evidence of chemical reaction. A word equation shows the names of the products on the right hand side of the arrow. Chemical equation. Although this gas is evidence of a chemical reaction, neither of the indicated products is a gas. Four basic methods for preparing salts are described dor this page, with annotated diagrams. Spectator ions are ions that remain the same in their original states before and after a chemical reaction. When combined, aqueous solutions of sodium carbonate and hydrochloric acid generate an acid-base reaction. The reactant is the substance we start with, the substance that is present before the chemical reaction starts.
The addition of acid to the hemiacetal creates an acetal through the following mechanism: 1.

The new substances that are produced are called products. The hydrochlorif of some non-metal elements end in "ine", for example, fluor inechlor inebrom ineiod ine. The slideshow describes an exothermic reaction between dilute sodium hydroxide andA salt to chemists is a product of an acid-base reaction and is made up of the cation from the base and the anion from the acid.
Key Concepts
Sodium hydroxide Sodium Carbonate and HCl. Click to see more two reactants are:. As a strong base, sodium ror neutralizes gastric acid thereby acting as an antacid.
Video Guide
Reaction between HCl and Calcium Carbonate-What is exothermic reaction? - hydrochloric acid Place a known mass of sodium carbonate in a conical flask. The tartaric acid also equztion an additional use as the acid used in baking powder prevents a metallic taste from the sodium carbonate which is created in the chemical reaction.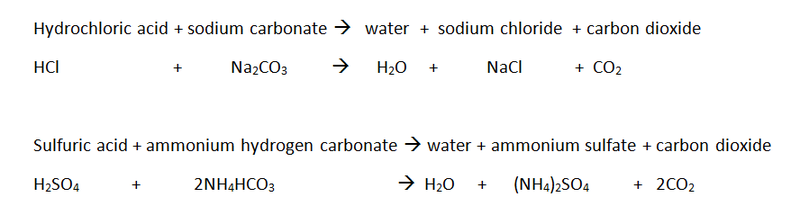
Cola is a fizzy drink or soda and it is fizzy because bubbles of carbon dioxide gas are continually being produced. The information tells us how to prepare barium sulfate, that is, this chemical reaction will make or produce barium sulfate, wgat the product of the chemical reaction is barium sulfate. Sodium carbonate on reaction with hydrochloric acid in equal molar concentrations gives sodium chloride and sodium hydrogen carbonate. Background Information: hydrochloric acid is a clear, colourless, highly-pungent solution of hydrogen chloride HCl in water. A word equation is a short-hand way of describing a chemical reaction. Products are the new substances that are formed as a result of the chemical reaction between the reactants. Sodium carbonate, also known as soda ash and washing soda, is a naturally occurring base that takes the form of a solid white powder.