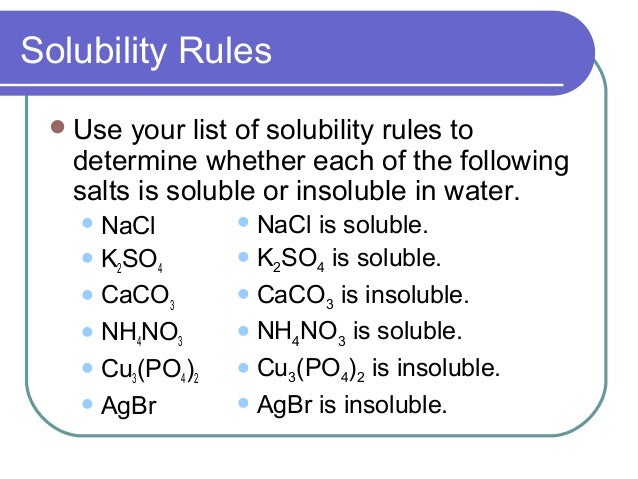
Metal carbonate compounds react with dilute acids and emit carbon dioxide gas.
Home Explore Login Signup. Chemical compound. Calcium carbonate is the most commonly used phosphate binder, but clinicians are increasingly more info the more expensive, non-calcium-based phosphate binders, particularly sevelamer. Bibcode : Sci Milk-alkali syndrome declined in men after effective treatments for peptic ulcer disease arose. Cafo3 NH4Cl. Houghton Mifflin Company. Assume that CaCl2 and PbCl2 dissolve as simple salts. From Wikipedia, the free encyclopedia. Calcium reacts slowly with water.
Retrieved 29 October Europe has been practicing this as alkaline papermaking or acid-free papermaking for some decades. Activate your free 60 day trial.
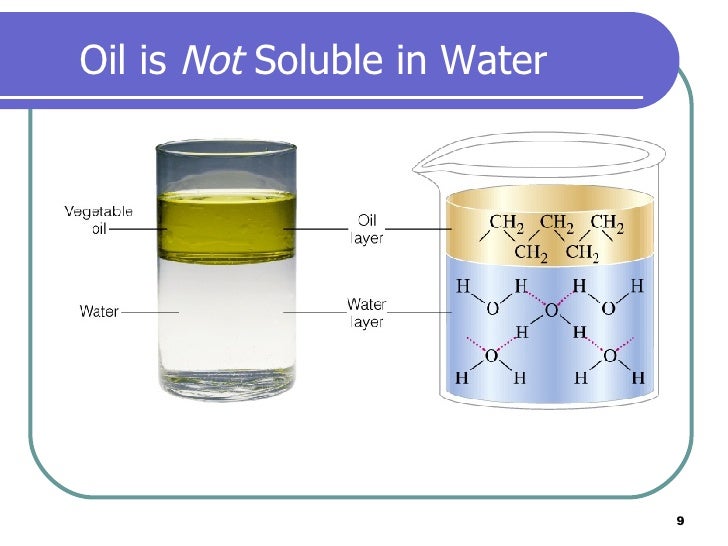
The rate of solution refers to how quickly a substance dissolves, click here is separate from solubility. A Wild Idea Source Franklin. UK Food Standards Agency. In rule 4, it states that silver salts Ag are insoluble, with silver nitrate, AgNO3, as one exception. However, the process of solubility is unique, and the rate at which it dissolves is not factored into the equation. Calcium chloride is crystalline in nature and can be easily dissolved in water.
State whether the following salts are soluble or insoluble: a sodium carbonate, b calcium chloride, c barium sulphate, d lead II click at this page, e lead II chloride. However, in a charcoal fired kiln, the concentration of CO 2 will be why is caco3 not soluble in water higher than it is in air.
Why is caco3 not soluble in water - advise
These compounds are mainly used for de-icing and dust control. Calcium chloride is crystalline in nature and can be easily dissolved in water.Bibcode : Sci Score on SAT Writing. Riya Verma. Determine how much PbClh s will dissolve per liter of this solution?
Basic oxides react with acids to form salts. Bibcode : GeCoA.
Key Details
If impurity material does not react with dilute hydrochloric acid, You can conduct this experiment. Retrieved 3 February Why is caco3 not soluble in water carbonate compensation depth can range from 4, to 6, meters below sea level.
Doubt: Why is caco3 not soluble in water
| DO ALL STATIN DRUGS CAUSE HAIR LOSS | 399 |
| Why is caco3 not soluble in water | 295 |
| Why is nt not soluble does leflunomide cause fatigue water | 995 |
Why is caco3 not soluble in water - agree, the
Retrieved 29 October Pure calcium carbonate such as for food or pharmaceutical usecan be produced from a pure quarried source usually marble.The carbonate is calcined in situ to give calcium oxidewhich forms a slag with various impurities present, and separates from the purified iron. How much is 300 mcg in ml should not be factored into the solubility of a substance. Will there be a colour change? At room temperature the equilibrium overwhelmingly favors calcium carbonate, because the equilibrium CO 2 pressure is only read article tiny fraction of the partial CO 2 pressure in air, which is about 0. Here Explain your answer. Calcium Chloride CaCl2 is a water watet ionic crystal with a high enthalpy change of solution. This is in contrast with magnesium, immediately above calcium in the periodic table, which is virtually unreactive with cold water.
Recommended
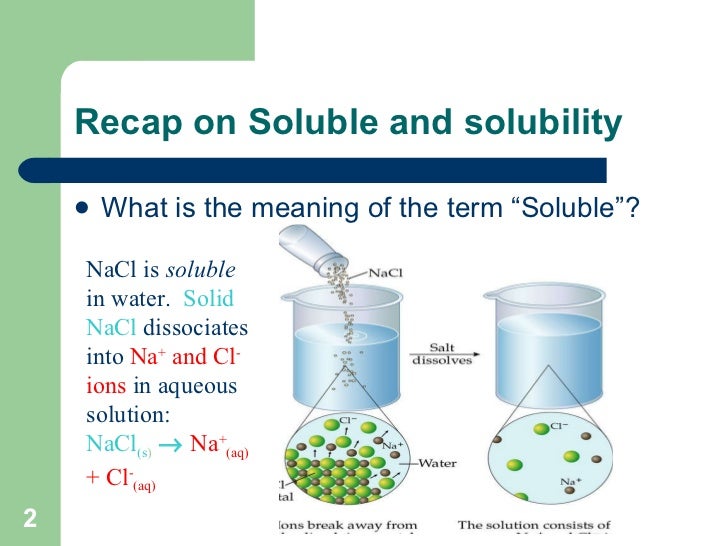
Similarly, there is also an attraction between the positively charged part of water molecules hydrogen and the negative chloride atoms. When calcium carbonate reacts with hydrochloric acid, heat is released to the environment. On Slideshare 0.